08 December 2022: Human Study
Use of Estimated Glomerular Filtration Rate and Urine Albumin-Creatinine Ratio Based on KDIGO 2012 Guideline in a Thai Community Hospital: Prevalence of Chronic Kidney Disease and its Risk Factors
Veeravan Lekskulchai1ACDEF*DOI: 10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176
Abstract
BACKGROUND: BACKGROUND Kidney disease is hard to detect at its early stage; therefore, the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guideline was developed for improving care and outcomes of patients with kidney disease. This study aimed to determine clinical outcomes from applying this guideline in a community hospital service. MATERIAL AND METHODS The patients’ data were extracted from their medical records and analyzed for outcomes of using the estimated glomerular filtration rate (eGFR) and urine albumin-creatinine ratio (UACR) for detecting kidney disease. RESULTS The eGFR was utilized in 36 172 patients aged ≥18 years, and 76.86% of them had normal kidney function. The prevalence of chronic kidney disease (CKD) was 8.20%; most patients (68%) with CKD were in stages 3a and 3b. The most common causes of CKD were diabetes and hypertension. The UACR was mainly used in patients with diabetes. The percentage of patients with UACR ≥3 mg/mmol creatinine alone was significantly higher than that of patients with eGFR <60 mL/min/1.73 m² alone in diabetes. Patients with controlled diabetes had a significantly higher percentage of normal kidney function and lower percentage of high UACR alone than patients with uncontrolled diabetes. CONCLUSIONS The prevalence and etiology of CKD in this region were similar to that of other regions. The KDIGO 2012 guideline helped identify CKD at the early stage. Most patients with diabetes in this region developed diabetic nephropathy in a classical pattern; thus, using eGFR concurrently with UACR could increase chances of detecting diabetic nephropathy at the early stage.
MATERIAL AND METHODS:
RESULTS:
CONCLUSIONS:
Keywords: Albuminuria, Diabetic Nephropathies, Glomerular Filtration Rate, kidney disease, diabetes, Humans, Adolescent, Adult, Creatinine, Prevalence, Hospitals, Community, Southeast Asian People, Kidney, Renal Insufficiency, Chronic, Risk Factors, Diabetes Mellitus, Albumins
Background
The glomerular filtration rate (GFR) is generally accepted as the best overall index of kidney function. It can be directly measured by the clearance of exogenous filtration markers or calculated by the clearance of endogenous filtration markers, such as creatinine. The direct measure of GFR, however, is too cumbersome for screening and ambulatory care; therefore, the estimated GFR (eGFR) calculated by serum creatinine (SCr) has become the most widely used method in clinical practice and epidemiologic research [1,2]. Currently, the eGFR can be acceptably calculated from either the Modification of Diet in Renal Disease (MDRD) study equation or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [3,4].
Kidney dysfunction or kidney disease is hard to be detected at the early stage because most patients do not have symptoms or have nonspecific symptoms. Diagnosis is commonly made after chance findings from screening tests, or the disease is detected only when symptoms are chronic. Without early detection and treatment, kidney disease can progress to end-stage kidney disease and death [5–7]. These are challenging health problems globally. Kidney Disease: Improving Global Outcomes (KDIGO) is an independent organization with the mission to improve care and outcomes of patients with kidney disease worldwide and has produced clinical practice guidelines in the field of kidney disease. The KDIGO 2012 Clinical Practice Guideline was developed for evaluation and management of chronic kidney disease (CKD) [5,6]. This guideline has been implemented globally, including in Thailand.
The KDIGO 2012 guideline classifies kidney disease into 6 stages, depending on the eGFR. Stages 1 and 2 have an eGFR ≥60 mL/min/1.73 m2 and stages 3a, 3b, 4, and 5 have an eGFR of 59 mL/min/1.73 m2 or lower [6]. Several studies have reported substantial kidney function decline with an eGFR <60 mL/min/1.73 m2. Accordingly, the guideline recommends that an eGFR <60 mL/min/1.73 m2 indicates decreased GFR and an eGFR <15 mL/min/1.73 m2 indicates kidney failure [6,8].
The urine albumin-creatinine ratio (UACR) has also been recommended as an index of kidney function. The KDIGO 2012 guideline states that a UACR between 3 and 30 mg/mmol creatinine (30–300 mg/g creatinine) is referred to as moderately increased, or microalbuminuria, and UACR >30 mg/mmol creatinine is termed severely increased [6]. In the combination of eGFR and UACR results, the KDIGO 2012 guideline has recommended the risk stratification of CKD as shown in the Table 1 [6]. Furthermore, the guideline defines CKD as abnormalities of kidney structure or function when there is persistently elevated UACR (≥3 mg/mmol creatinine), persistently reduced eGFR (<60 ml/min per 1.73 m2), or both for greater than 3 months and suggests assessing eGFR and UACR at least annually in patients with CKD [6,9].
To reduce the prevalence of CKD, its common causes should be defined. In developed countries, hypertension and diabetes are the most frequent causes of CKD, but in other countries, different causes of CKD, such as infections, glomerulonephritis, and herbal and environmental toxins, can be as frequent as the causes of hypertension and diabetes or coexist with them [10,11].
This study aimed to investigate clinical outcomes from applying the KDIGO 2012 Clinical Practice Guideline in a community hospital service in Thailand. The prevalence of CKD and its common risk factors in this community were also investigated.
Material and Methods
The study was conducted at the HRH Princess Maha Chakri Sirindhorn Medical Center, Nakhon Nayok, Thailand. The study protocol was approved with a waiver of informed consent by the Srinakharinwirot University Ethics Committee for Human Research. Analyses were conducted using the electronic medical records of patients admitted to the hospital from January 2016 to December 2018, before the COVID-19 pandemic. All records of patients aged ≥18 years and having SCr laboratory results were collected anonymously and then coded by new identification numbers. Data on patient sex and age, admission date, diagnosis, time laboratory tests were ordered, and the laboratory tests and their results were extracted from the records. The average ages were calculated from the patients’ age at their first data collected during the study period.
The creatinine level was measured using an enzymatic method, while urinary albumin and hemoglobin A1C (HbA1C) levels were measured using turbidimetric immunoassays. All were performed on the Architect ci8200 instrument (Abbott Laboratories, Abbott Park, IL, USA). The eGFR and UACR were automatically calculated by the laboratory information system. The equation used to calculate eGFR using MDRD was as previously reported [12] as eGFR (MDRD)=186.3×age−0.203×(SCr)1.154 (if female×0.742) when SCr is in mg/dL. The eGFR using CKD-EPI was calculated by equations recommended by the Nephrology Society of Thailand in 2016 (not published) as follows:
All concurrently available eGFR (MDRD) and eGFR (CKD-EPI) were compared for GFR categorization using a 6×6 cross-tabulation. For patients with multiple SCr measurements, the available eGFR from their final episode was used to stage kidney function. All patients with eGFR (CKD-EPI) <60 mL/min/1.73 m2 persistently over 3 months without a recovery were counted as having CKD. End-stage kidney disease (ESKD) was counted from patients diagnosed with CKD stage 5 along with a report of eGFR <15 mL/min/1.73 m2 or having dialysis and patients with other diagnoses along with eGFR <15 mL/min/1.73 m2 persistently over 6 months without a recovery. When eGFR and UACR were available simultaneously, the kidney function was classified as either normal (eGFR ≥60 mL/min/1.73 m2 and UACR <3 mg/mmol creatinine), albuminuria only (eGFR ≥60 mL/min/1.73 m2 and UACR ≥3 mg/mmol creatinine), low eGFR only (eGFR <60 mL/min/1.73 m2 and UACR <3 mg/mmol creatinine), or both albuminuria and low eGFR (eGFR <60 mL/min/1.73 m2 and UACR ≥3 mg/mmol creatinine). HbA1C, when available, was used to determine the glycemic control of patients with diabetes as controlled (HbA1C ≤6.5%) or uncontrolled (HbA1C >6.5%). Statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Intergroup comparisons were made using the chi-square test. Statistical significance was defined as
Results
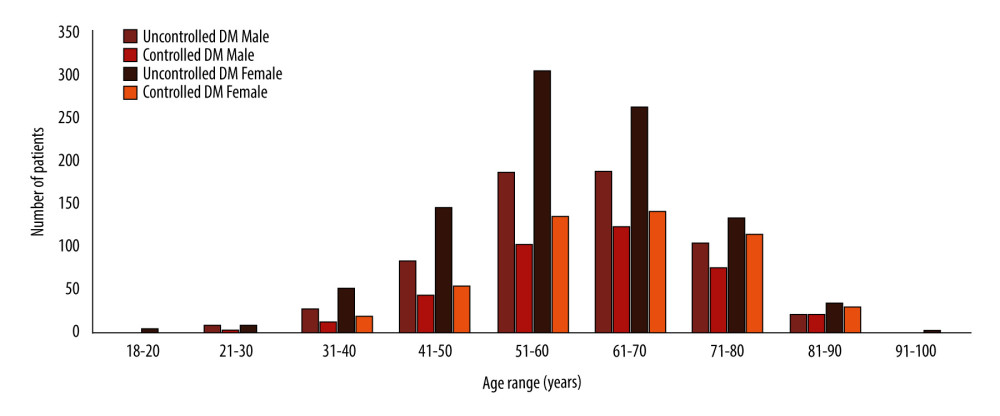
From 2016 to 2018, laboratory tests were obtained from 62 780 patients aged ≥18 years (Table 2). SCr results were obtained in 57.62% of patients, and UACR along with SCr in 5.29% of patients. The UACR was utilized mostly in patients with diabetes (60.77%). The results of HbA1C along with UACR were available for 2481 patients with diabetes. Over half of them (65.18%) were defined as uncontrolled diabetes or having HbA1C >6.5% (Table 2). Additionally, the number of patients with uncontrolled diabetes was greater than the number of patients with controlled diabetes for both sexes and all age groups (Figure 1).
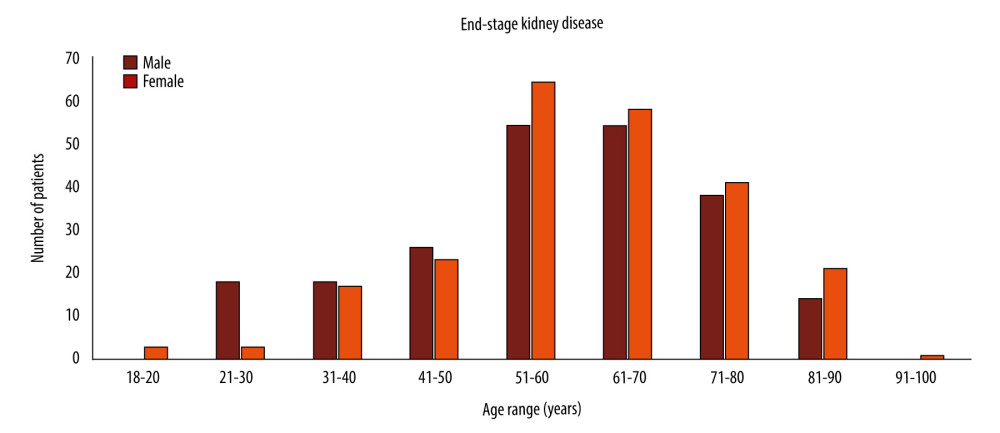
The eGFR (MDRD) and eGFR (CKD-EPI) were concurrently reported from 138 417 serum samples, with the 2 eGFR equations indicating the same stage of kidney disease in 88.42% of samples (Table 3). Disagreement was highly seen at the eGFR ≥60 mL/min/1.73 m2, at 6.39%. Compared with the eGFR (CKD-EPI) at values <60 mL/min/1.73 m2, the eGFR (MDRD) under-staged in 5.07% and over-staged in 0.14% of patients. As shown in Table 4, 76.86% of total patients tested for SCr had eGFR (CKD-EPI) ≥60 mL/min/1.73 m2 at their latest episode, and CKD was detected in 8.20% of patients. Most patients with CKD (68%) were in stages 3a and 3b. Diabetes and non-diabetes hypertension were the most common causes of CKD. ESKD was detected in 447 patients (Table 4), who were in all age groups, but most were in the groups including patients from 51 to 70 years of age (Figure 2). Most patients with ESKD had no possible cause reported. At ages ≤30 years, the ESKD was identified in 14 male and 4 female patients. Infections were the most common cause of ESKD in these young patients, in 7 male patients and 1 female patient.
In all patients with eGFR and UACR data concurrently available, 52.44% had normal kidney function, 24.79% had albuminuria alone, 9.22% had low eGFR alone, and 13.55% had both albuminuria and low eGFR (Table 5). Likewise, in 2588 patients with diabetes tested for both eGFR and UACR, 51% had normal kidney function, 26.97% had albuminuria alone, 8% had low eGFR alone, and 14.03% had albuminuria and low eGFR. In both sexes, the percentages of patients with albuminuria alone were significantly higher than that of those with low eGFR alone. Men without diabetes had significantly higher percentages of low eGFR alone than did women without diabetes. Also, the percentage of low eGFR alone was significantly higher in men without diabetes than in men with diabetes. In diabetes, sex had no significant impact on declining kidney function. Albuminuria alone was significantly higher in patients with diabetes than in patients without diabetes. The percentage of patients with albuminuria alone was significantly higher than the percentage of patients with low eGFR alone in women without diabetes, with diabetes, and in controlled and uncontrolled diabetes in both sexes. There was a significantly higher percentage of normal kidney function in patients with controlled diabetes and a lower percentage of albuminuria than in patients with uncontrolled diabetes (Table 6).
Discussion
According to the systematic analysis of the Global Burden of Disease Study, approximately 1.23 million people died from CKD in 2017, and the global all-age mortality rate for CKD increased by 41.5% between 1990 and 2017 [13]. Early detection of CKD is critical to slow disease progression, prevent long-term morbidity and mortality, and decrease health care spending [14]. In the present study population, SCr and eGFR were highly utilized (Table 2) not only for detecting and monitoring CKD but also for other clinical purposes as recommended [15], including screening for acute kidney injury in various situations and assessment of kidney function in order to determine whether patients could tolerate imaging contrast or whether certain nephrotoxic medicines were safe to administer. Consequently, most patients (76.86%), as expected, had normal kidney function, ensuring further clinical management. Nevertheless, the present percentage of patients with normal kidney function was lower than that seen in Korea [16]. As previously reported [17], CKD has a high rate of occurrence in low- and middle-income countries, including Thailand. From assessing the precision of eGFR from CKI-EPI and MDRD equations in staging kidney function, it was found that they were highly agreeable (88.42%) (Table 3). Also, according to previous reports [11,18,19], disagreement between them occurred mostly when eGFR ≥60 mL/min/1.73 m2. Thus, both could be used to detect and monitor abnormal kidney function and CKD.
Determining CKD based on the KDIGO 2012 guideline was complicated because, in many cases, patients were admitted for various illnesses during 3 successive months and some recovered after several months. As previously reported, kidney disease has numerous complex causes and CKD in patients with diabetes is heterogeneous, caused by multiple processes [9,20]. The guideline describes that the recovery of CKD in some patients could be entirely reversible, either spontaneously or with treatment [6]. In the present study, recovery could even be seen in some patients with eGFR <15 mL/min/1.73 m2. These results led to unclear interpretation, and subsequently other more invasive and expensive tests, such as kidney biopsies, were conducted to make an accurate diagnosis.
The common causes of CKD in the present study were diabetes and non-diabetes hypertension (Table 4), which are similar to that reported in developed countries [10,11]. Also, non-diabetes non-hypertensive cardiovascular diseases, infections, cancers, autoimmune diseases, and kidney and urinary tract disorders were common causes of CKD in this study population (Table 4). In these situations, fast detection and treatment seemed to be key factors for preserving the kidney. According to previous studies, systemic as well as local inflammation and renal hemodynamic changes have been identified as important processes in the development of renal complications [16,21,22]. Because of the high cost of service, the UACR was rarely monitored in the present study population and, thus, persistently elevated UACR for greater than 3 months was infrequently available. Thus, the prevalence of CKD in the present study was determined based upon eGFR only, which was 8.20%, in accordance with previous reports [23,24]. The present study used data before the COVID-19 pandemic to avoid the pandemic’s impact on the accuracy of CKD prevalence. As recently reported [25,26], the health care services in Thailand were disrupted, and there were unusual high mortality rates from either COVID-19 infection or other diseases combined with COVID-19 infection during the pandemic.
ESKD in this study was detected in 5.3% of patients with CKD, which was high in a group solely diagnosed CKD stage 5. From the limited available data, a possible cause of ESKD in this group was not identified. As reported [6,27], the cause of kidney disease might not be known, since the progression of CKD is often slow and there are few specific symptoms until the disease is very advanced. Diagnosis is commonly made after chance findings from screening tests. The most common identified cause of ESKD in the present study was non-diabetes hypertension, followed by diabetes. Moreover, according to a report [20], infections including pneumonia, tuberculosis, AIDS, and viral hepatitis could lead to ESKD, particularly in young men, which might result from the infection itself or from adverse effects of drugs taken.
As previously reported [28], the UACR was rarely utilized alone but was frequently used along with SCr due to physicians’ familiarity with SCr. However, the rate of utilizing the UACR along with SCr was not high (Table 2). The main reason was its high cost, around 10 times higher than that of SCr. Therefore, physicians used UACR limitedly, preferring not to use it as a screening test or to measure follow-up values unnecessarily. Deprived access to laboratory services, as suggested [20], may lead to underestimating the true burden posed by kidney disease. It was expected that the prevalence of CKD might have been higher than 8.20% if UACR was monitored and interpreted along with eGFR.
In the KDIGO 2012 guideline, albuminuria is the earliest marker of glomerular diseases, where it generally appears before the reduction in GFR, and it is associated with the duration and severity of hypertension [6]. In this community, the UACR was highly utilized in diabetes but not often in non-diabetes hypertension (Table 2): compared to the SCr utilization, only 60.77% and 9.74% of the diabetes and non-diabetes patients, respectively, were tested for UACR. As mentioned above, diabetes and hypertension were the common identified causes of ESKD; thus, increasing UACR screening in diabetes and hypertension patients may help reduce the incidence of ESKD.
Monitoring both albuminuria and eGFR over time helps identify individuals with diabetes at high risk of kidney failure and those who require close monitoring for early initiation of appropriate preventive and therapeutic strategies [29]. The classical description of diabetic nephropathy is a slow and progressive increase in albuminuria, followed later in the disease by a decrease in eGFR [3,30]. Nevertheless, several studies have reported substantial kidney function decline (eGFR <60 mL/min/1.73 m2) in patients with diabetes without albuminuria [3,8,16,29,30]. In the present study, almost all patients with diabetes tested for UACR were also tested for HbA1C, and most of them in both sexes and all age groups were unable to control their blood glucose, which can be a global health issue.
Based on the UACR and eGFR, low eGFR alone was prominently seen in men without diabetes but not in women without diabetes. Non-diabetes hypertension was the most common cause in these groups; thus, the cause of kidney dysfunction might not be an explanation of this finding. Regarding the similar rates of ESKD in both sexes, delayed kidney disease detection could also not explain this finding. In diabetes, sex had no impact on declining kidney function (Table 6). According to previous reports [3,31], in this community, around 1 in 5 patients with diabetes (22.08%) had low eGFR and approximately 40.65% had albuminuria. Although low eGFR alone was detected in patients with diabetes, the percentages were significantly lower than that of albuminuria alone in both sexes and with controlled and uncontrolled diabetes (Table 6). These results were also seen in women without diabetes. Therefore, the classic diabetic nephropathy was actually often detected, and UACR could help detect kidney disease at the early stage in clinical practices.
Patients with controlled diabetes had a significantly higher rate of normal kidney function than did patients with uncontrolled diabetes. Moreover, patients with controlled diabetes had significantly lower percentages of albuminuria alone and both albuminuria and low eGFR than did those with uncontrolled diabetes. This indicated that good glycemic control could more or less help prevent or delay the development of diabetic nephropathy. Overall, the results support that UACR can be an early indicator of CKD progression and complications beyond eGFR, as previously recommended [27,32]. Therefore, all patients with diabetes and hypertension should have their renal function screened at least annually from the time of diagnosis using the UACR and eGFR [31]. Additionally, intensive glycemic control, optimization of blood pressure, and the use of renal protective drugs can slow or stop progression of diabetic nephropathy [3]. Effectively controlled blood pressure is also necessary to prevent the development of CKD and ESKD.
The World Health Organization’s Action Plan for non-communicable diseases states that a rise in the number of patients with kidney disease results from lack of early detection and management of hypertension and diabetes [33]. Furthermore, limited epidemiological data, common lack of awareness, and frequent poor access to laboratory services can result in the underestimation of the true burden posed by kidney disease [20]. Kidney disease itself may not be a leading cause of death worldwide but it has an indirect impact on global morbidity and mortality by increasing the risks associated with diabetes, hypertension, cardiovascular diseases, and infection. Therefore, causes, consequences, and costs of kidney disease should be investigated and suggested for national and public health policy in all countries [20]. The KDIGO 2012 guideline recommends that decisions on screening and referral strategies could have a major impact on the costs and quality of health-care and encouraged local initiatives combined with national policy and practice changes to improve outcomes of patients with CKD [6]. In Thailand, a middle income country, there are limited resources and budget for health care services and a limited number of nephrologists. The nation has adopted peritoneal dialysis as a potentially cheap, accessible, and sustainable mode of delivering renal replacement therapy since 2008 [34], and from February 2022 the cost for peritoneal dialysis has been fully covered for Thai people by the national health care policy [35,36]. However, the laboratory cost for screening for kidney disease is still uncovered by the national policy, probably because the effectiveness of UACR in screening for early kidney disease is still ambiguous. To reduce the prevalence of CKD, more supportive evidences for the effectiveness of UACR are required; otherwise, a more accurate test with a reasonable cost should be developed and marketed for replacing the UACR.
With its retrospective design, this study had some limitations. First, the available data were limited, and under the protocol approved by the research ethics committee, seeking information before and after the study period was not allowed. Second, the COVID-19 pandemic interfered with the accuracy of the epidemiological data; thus, this study used information before the pandemic, which was expected to be more precise and similar to data after the COVID-19 pandemic. Last, the study was conducted in a community in Thailand that may not have been representative of the whole nation, although it was expected to not be much different.
Conclusions
By applying the KDIGO 2012 Clinical Practice Guideline in this hospital service, a CKD prevalence of 8.20% was found. The most common cause of CKD was diabetes, followed by non-diabetes hypertension. The ESKD was detected in 5.3% of patients with CKD, mostly without specified cause. Hypertension and diabetes were the most common identified causes of ESKD. UACR was highly used in diabetes but, compared with the SCr, it was far less utilized in clinical practice. The percentage of albuminuria alone in patients with diabetes was statistically significantly higher than that of low eGFR alone. Regardless of its high service cost, UACR is a useful tool in screening for early kidney dysfunction, and screening for UACR may help reduce the incidence of ESKD.
Tables
Table 1. Risk stratification of chronic kidney disease by eGFR and UACR [6].![Risk stratification of chronic kidney disease by eGFR and UACR [6].](https://jours.isi-science.com/imageXml.php?i=t1-medscimonitbasicres-28-e938176.jpg&idArt=938176&w=1000) Table 2. Demographics of the study patients.
Table 2. Demographics of the study patients.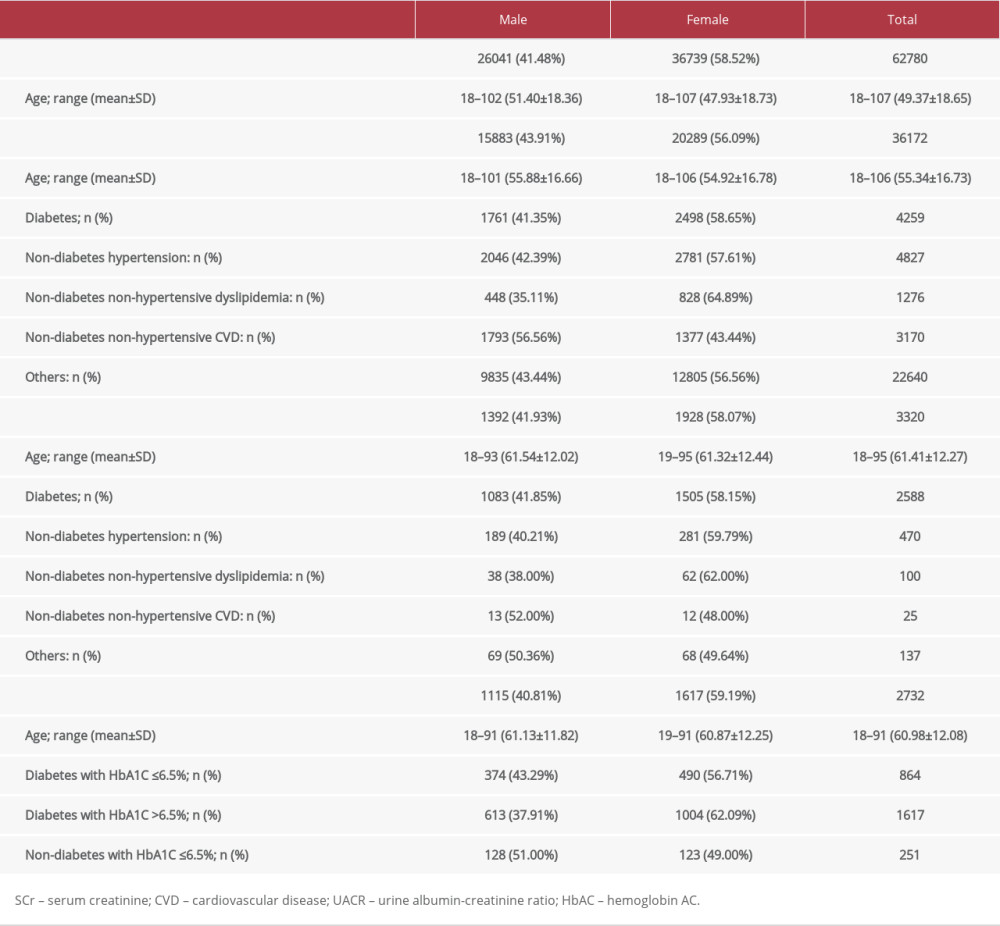 Table 3. Agreement of eGFR (MDRD) and eGFR (CKD-EPI) for GFR categorization.
Table 3. Agreement of eGFR (MDRD) and eGFR (CKD-EPI) for GFR categorization.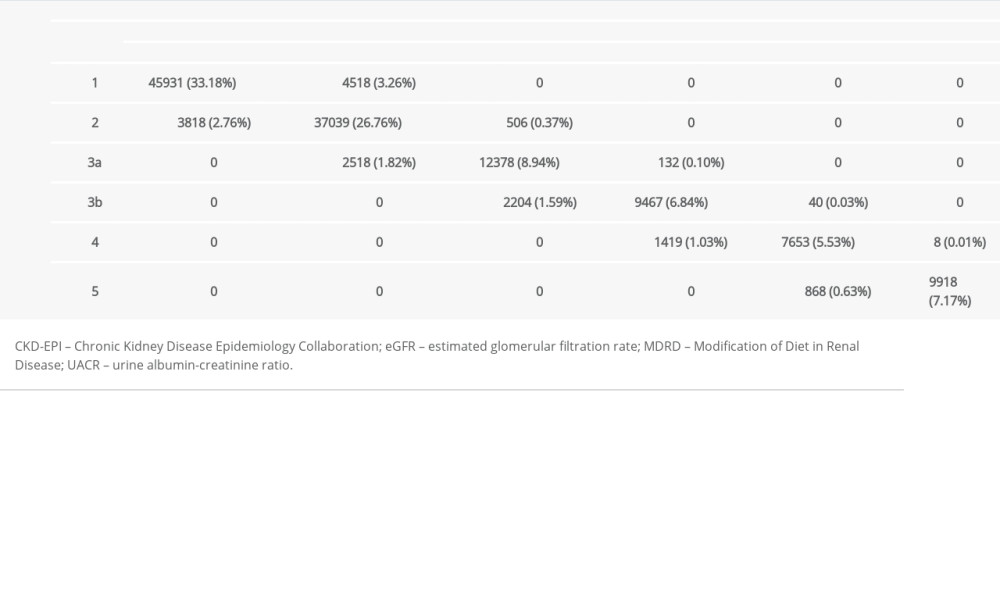 Table 4. Patients with chronic kidney disease and end-stage kidney disease by sex.
Table 4. Patients with chronic kidney disease and end-stage kidney disease by sex.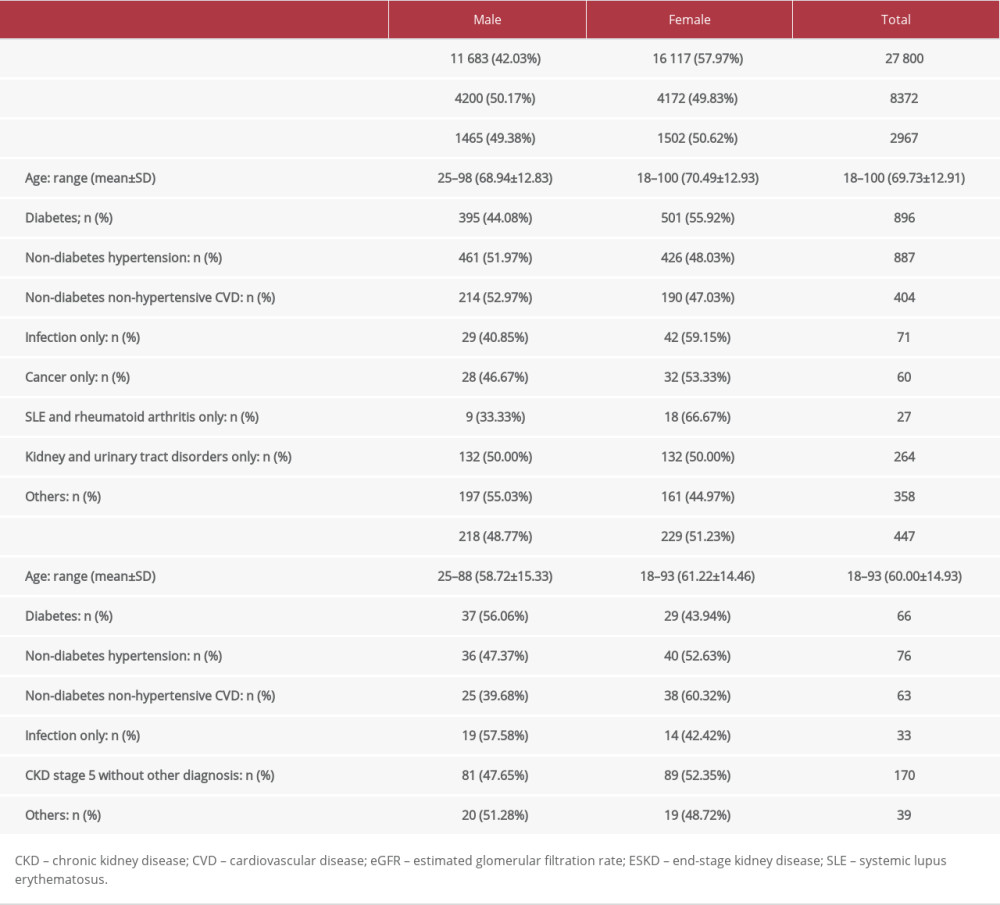 Table 5. Kidney function based on eGFR and UACR.
Table 5. Kidney function based on eGFR and UACR.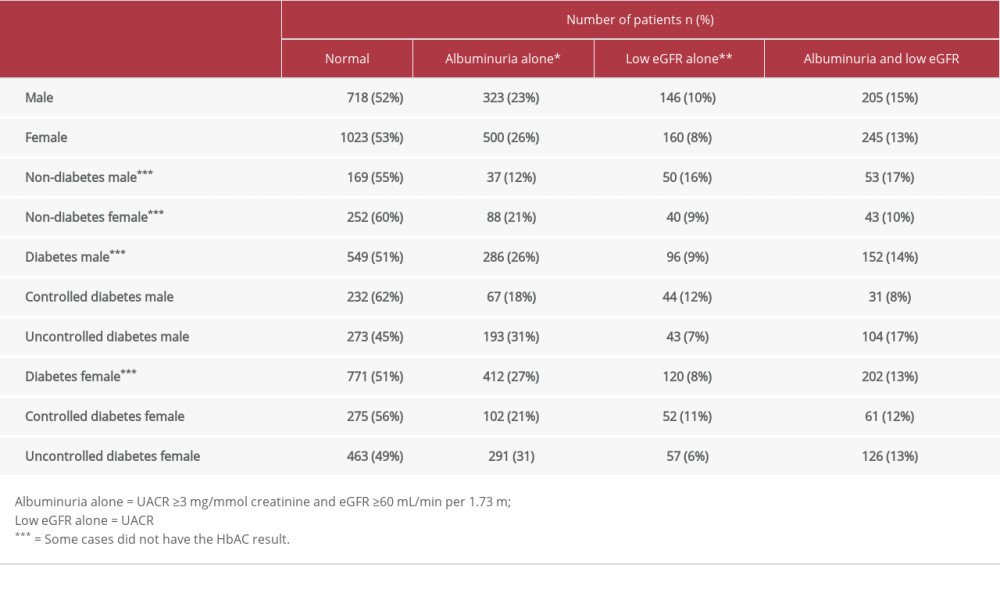 Table 6. Comparative results of kidney function between certain groups.
Table 6. Comparative results of kidney function between certain groups.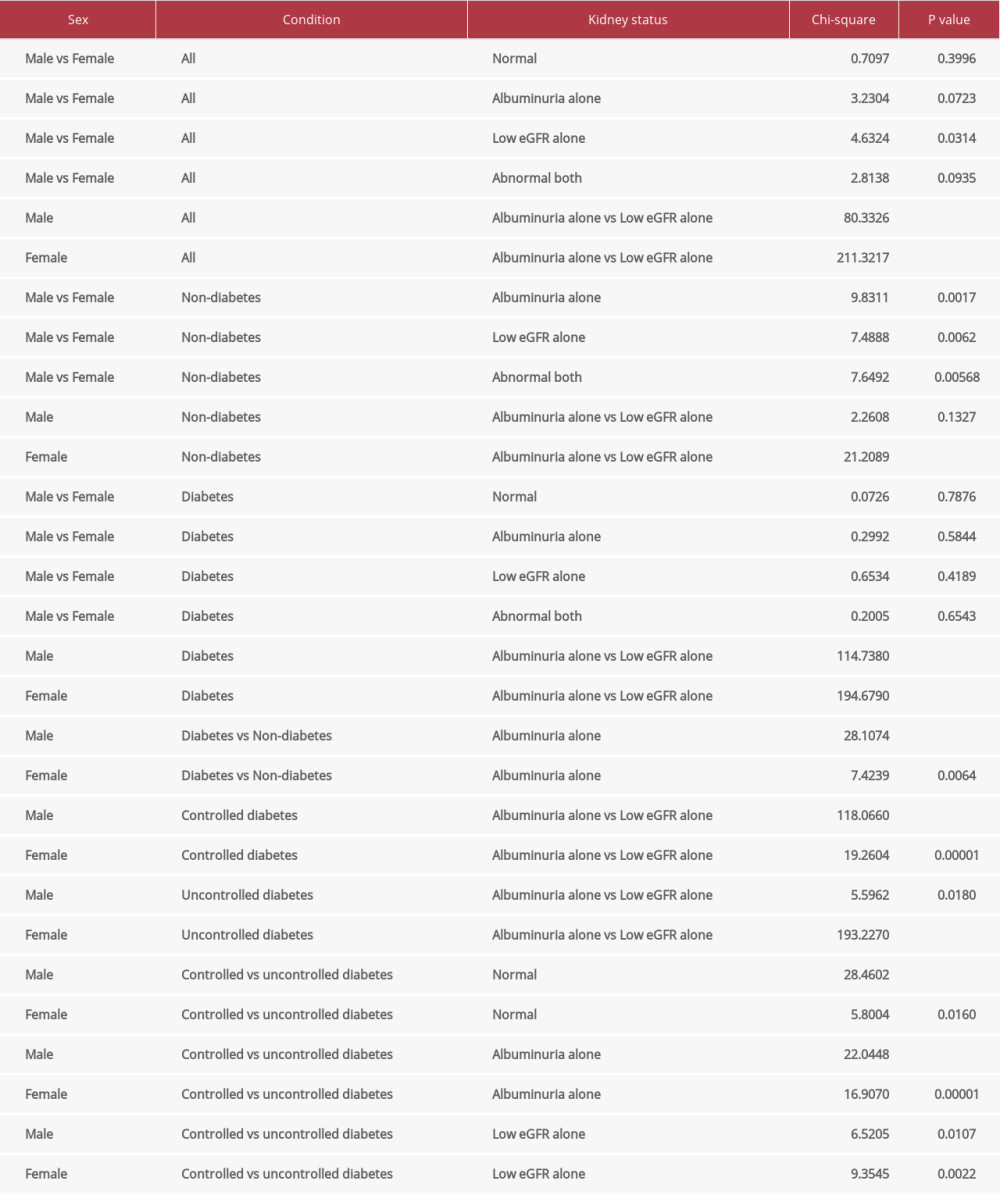
References
1. Bjornstad P, Karger AB, Maahs DM, Measured GFR in routine clinical practice-the promise of dried blood spots: Adv Chronic Kidney Dis, 2018; 25(1); 76-83
2. Levey AS, Coresh J, Tighiouart H, Measured and estimated glomerular filtration rate: Current status and future directions: Nat Rev Nephrol, 2020; 16; 51-64
3. McFarlane P, Cherney D, Gilbert RE, Senior PDiabetes Canada Clinical Practice Guidelines Expert Committee, 2018 Clinical practice guidelines chronic kidney disease in diabetes: Can J Diabetes, 2018; 42; S201-9
4. Kilbride HS, Stevens PE, Eaglestone G, Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly: Am J Kidney Dis, 2013; 61(1); 57-66
5. Levin A, Stevens PE, Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward: Kidney Inter, 2013; 85; 49-61
6. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease: Kidney Inter, 2013; 3(Suppl); 1-150
7. Webster AC, Nagler EV, Morton RL, Masson P, Chronic kidney disease: Lancet, 2017; 389(10075); 1238-52
8. Koye DN, Magliano DJ, Reid CM, Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: The CRIC (Chronic Renal Insufficiency Cohort) study: Am J Kidney Dis, 2018; 72(5); 653-61
9. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group, KDIGO 2020 Clinical practice guideline for diabetes management in chronic kidney disease: Kidney Int, 2020; 98(4S); S1-115
10. Alicic RZ, Rooney MT, Tuttle KR, Diabetic kidney disease: Challenges, progress, and possibilities: Clin J Am Soc Nephrol, 2017; 12; 2032-45
11. Jha V, Garcia-Garcia G, Iseki K, Chronic kidney disease: Global dimension and perspectives: Lancet, 2013; 382(9888); 260-72
12. The Australasian Creatinine Consensus Working Group, Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: A position statement: Med J Aus, 2005; 183; 138-41
13. GBD Chronic Kidney Disease Collaboration, Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017: Lancet, 2020; 395(10225); 709-33
14. Gaitonde DY, Cook DL, Rivera IM, Chronic kidney disease: Detection and evaluation: Am Fam Physician, 2017; 96(12); 776-83
15. Berg AH, Eyk JV, Which methods for determining glomerular filtration rate most strongly associate with risk of progression of kidney disease?: Clin Chem, 2019; 65(3); 361-62
16. Ji E, Kim YS, Prevalence of chronic kidney disease defined by using CKD-EPI equation and albumin-to-creatinine ratio in the Korean adult population: Korean J Intern Med, 2016; 31; 1120-30
17. Stanifer JW, Muiru A, Jafar TH, Patel UD, Chronic kidney disease in low- and middle-income countries: Nephrol Dial Transplant, 2016; 31; 868-74
18. Kitiyakara C, Yamwong S, Vathesatogkit P, The impact of different GFR estimating equations on the prevalence of CKD and risk groups in a Southeast Asian cohort using the new KDIGO guidelines: BMC Nephrol, 2012; 13; 1
19. Levey AS, Inker LA, Coresh J, GFR estimation: From physiology to public health: Am J Kidney Dis, 2014; 63(5); 820-34
20. Luyckx VA, Tonelli M, Stanifer JW, The global burden of kidney disease and the sustainable development goals: Bull World Health Organ, 2018; 96; 414-22
21. Persson F, Frimodt-Møller M, Rossing P, Inflammation leads the way on the ROADMAP to diabetic kidney disease: Kidney Int Rep, 2019; 4; 1362-65
22. Chang Y-S, Li Y-H, Lee I-T, A synergistic effect of variability in estimated glomerular filtration rate with chronic kidney disease on all-cause mortality prediction in patients with type 2 diabetes: A retrospective cohort study: Cardiovasc Diabetol, 2021; 20; 209
23. Kanjanabuch T, Takkavatakarn K, Global dialysis perspective: Thailand: Kidney360, 2020; 1; 671-75
24. Hill NR, Fatoba ST, Oke JL, Global prevalence of chronic kidney disease – a systematic review and meta-analysis: PLoS One, 2016; 11(7); e0158765
25. Shiels MS, Haque AT, Berrington de González A, Freedman ND, Leading causes of death in the US during the COVID-19 pandemic, March 2020 to October 2021: JAMA Intern Med, 2022; 182(8); 883-86
26. Kuehn BM, Reduced HIV testing and diagnoses during COVID-19 pandemic: JAMA, 2022; 328(6); 519
27. Carrero JJ, Grams ME, Sang Y, Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality: Kidney Inter; 91; 244-51 201
28. Lekskulchai V, Appropriateness of using tests for blood glucose and diabetic complications in clinical practice: Experiences in a hospital in Thailand: Med Sci Monit, 2018; 24; 7382-86
29. Oshima M, Toyama T, Hara A, Combined changes in albuminuria and kidney function and subsequent risk for kidney failure in type 2 diabetes: BMJ Open Diab Res Care, 2021; 9; e002311
30. Chena Y, Lee K, Ni Z, He JC, Diabetic kidney disease: Challenges, advances, and opportunities: Kidney Dis, 2020; 6; 215-25
31. Thomas MC, Brownlee M, Susztak K, Diabetic kidney disease: Nat Rev Dis Primers, 2020; 1; 15018
32. Heerspink HJL, Greene T, Tighiouart H, Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials: Lancet Diabetes Endocrinol, 2019; 7; 128-39
33. World Health Organization: Action plan for the prevention and control of non-communicable diseases, 2013–2020, 2013, Geneva, Switzerland, World Health Organization
34. Morad Z, Choong HL, Tungsanga K, Funding renal replacement therapy in Southeast Asia: Building public-private partnerships in Singapore, Malaysia, Thailand, and Indonesia: Am J Kidney Dis, 2015; 65; 799-805
35. The National Health Security Office, Guidelines for obtaining service expenses for chronic renal disease patients: Guidelines for obtaining health care expenses, 2019; 163-64, Nonthaburi, Sahamit Printing and Publishing
36. Kanjanabuch T, Pongpirul K, Peritoneal dialysis care during the COVID-19 pandemic, Thailand: Bull World Health Organ, 2022; 100(2); 155-60
Figures
Tables
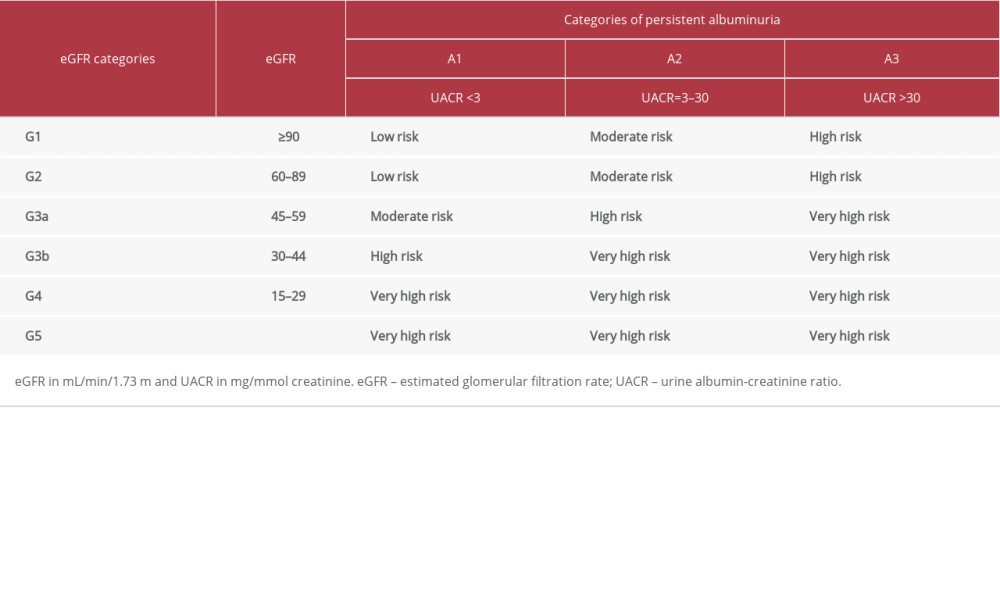 Table 1. Risk stratification of chronic kidney disease by eGFR and UACR [6].
Table 1. Risk stratification of chronic kidney disease by eGFR and UACR [6]. Table 2. Demographics of the study patients.
Table 2. Demographics of the study patients. Table 3. Agreement of eGFR (MDRD) and eGFR (CKD-EPI) for GFR categorization.
Table 3. Agreement of eGFR (MDRD) and eGFR (CKD-EPI) for GFR categorization. Table 4. Patients with chronic kidney disease and end-stage kidney disease by sex.
Table 4. Patients with chronic kidney disease and end-stage kidney disease by sex. Table 5. Kidney function based on eGFR and UACR.
Table 5. Kidney function based on eGFR and UACR. Table 6. Comparative results of kidney function between certain groups.
Table 6. Comparative results of kidney function between certain groups. Table 1. Risk stratification of chronic kidney disease by eGFR and UACR [6].
Table 1. Risk stratification of chronic kidney disease by eGFR and UACR [6]. Table 2. Demographics of the study patients.
Table 2. Demographics of the study patients. Table 3. Agreement of eGFR (MDRD) and eGFR (CKD-EPI) for GFR categorization.
Table 3. Agreement of eGFR (MDRD) and eGFR (CKD-EPI) for GFR categorization. Table 4. Patients with chronic kidney disease and end-stage kidney disease by sex.
Table 4. Patients with chronic kidney disease and end-stage kidney disease by sex. Table 5. Kidney function based on eGFR and UACR.
Table 5. Kidney function based on eGFR and UACR. Table 6. Comparative results of kidney function between certain groups.
Table 6. Comparative results of kidney function between certain groups. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176