29 June 2020: Clinical Research
Clinical Utilization of Blood and Urine Cultures and Incidences of Bacteremia and Bacteriuria in a Hospital in Thailand
Veeravan Lekskulchai1ACDEF*DOI: 10.12659/MSMBR.924204
Med Sci Monit Basic Res 2020; 26:e924204
Abstract
BACKGROUND: To effectively treat sepsis and urinary tract infection (UTI), blood and urine cultures should be used appropriately and relative to incidences of bacteremia and bacteriuria. This study aimed to investigate the use of blood and urine cultures and incidences of bacteremia and bacteriuria in a hospital in Thailand.
MATERIAL AND METHODS: Medical records of patients admitted from 2016 to 2018 were randomly selected and data in the records were anonymously extracted for investigation.
RESULTS: From 12 000 records, data on blood and urine cultures were extracted from 9% and 4% of them, respectively. The negative rate of blood culture was 87.48%. Bacteremia was detected in 10.22%. The positive rate of urine culture was 27.38% and the contamination rate was 31.26%. Escherichia coli was the most common cause of community-onset bacteremia and bacteriuria. Methicillin-resistant coagulase-negative staphylococci and Acinetobacter baumannii were the most common cause of hospital-acquired bacteremia while yeasts were the most common cause of hospital-acquired UTI.
CONCLUSIONS: A high negative rate of blood culture may result not only from its low sensitivity but also from liberal test use to identify sepsis in some conditions. Improper urine collection is the main problem with use of urine culture.
Keywords: Bacteremia, Bacteriuria, Clinical Laboratory Techniques, Sepsis, Anti-Bacterial Agents, Blood Culture, Cohort Studies, Hospitals, Incidence, Thailand, Urine Specimen Collection
Background
Blood culture is a clinical laboratory test used to detect bacteremia in patients suspected of having sepsis. Due to the dangers of under treatment and high mortality associated with bacteremia and the concern about using inappropriate antibiotics, blood cultures are expected to be liberally used [1,2]. However, as a culture-based method, blood culture has some limitations, including low sensitivity and long time to positivity [3,4]. The results may be negative in patients with sepsis because of a very low number of circulating microbes, fastidious microorganisms, uncultivable organisms, and antibiotic treatment initiated before blood sampling [3–6]. Consequently, use of blood culture for sepsis may be limited and identification of the condition may rely on clinical signs and other more rapid tests, including complete blood count and serum lactate. Indeed, sepsis has recently been defined as presence of fever, leukocytosis (white blood cell [WBC] >12,000 cells/mm3) or leucopenia (WBC <4,000 cells/mm3), neutrophilia (neutrophils ≥80%), and hyperlactatemia (serum lactate >2 mmol/L) [5–8].
Bacteremia may be detected from the blood of patients collected within 48 hours after admission, which is called community-onset bacteremia [9,10]. Sources of infection in this bacteremia may be severe localized or systemic infections, which cause bacteria to enter the bloodstream through the lymphatic system [11]. Some hospitalized patients may get an infection including sepsis while receiving medical care because of their illness and compromised immune system, and blood culture performed more than 48 h after admission may be positive, which is called hospital-acquired bacteremia [12]. Unlike community-onset bacteria, the main sources of infection in hospital-acquired bacteremia are an invasive device, particularly central line catheters, and some invasive procedures [12,13].
Urine cultures are a useful tool for identifying bacteriuria in patients suspected of having urinary tract infection (UTI). Although not a life-threatening condition, UTI is among the most common bacterial infections acquired in both the community and hospitals [14,15]. In addition, urine can be collected easily and noninvasively. For these reasons, urine culture also may be extensively used. It does not, however, produce rapid results because of the time required for organisms to grow on specific media [5], which may restrict its utilization. Clinicians may use urinalysis to predict bacteriuria before turning to urine culture. However, the association between results of urinalysis, specifically nitrite and leucocyte esterase [LE], and bacteriuria is still in doubt. Some reports indicate that urinalysis is not a reliable predictor of bacteriuria [16,17], whereas others suggest that urine may not be necessarily obtained for culture if the LE and nitrite are negative [18,19]. Like bacteremia, bacteriuria can be either community-onset or hospital-acquired, depending on the timing of detection of bacteriuria after patients are admitted to the hospital. A previous report indicates that prevalence of community-onset bacteriuria is high in women and the elderly whereas hospital-acquired bacteriuria is detected frequently in hospitalized patients who have been catheterized for at least 24 hours during their hospital stay [20].
This study aimed to investigate use of blood and urine cultures and incidences of bacteremia and bacteriuria in a hospital in Thailand.
Material and Methods
This retrospective study was approved with a waiver of informed consent by the Srinakharinwirot University Ethics Committee for Human Research. It was conducted at the HRH Princess Maha Chakri Sirindhorn Medical Center, Nakhon Nayok, Thailand. Medical records of patients admitted from January 2016 to December 2018 were sorted by their admission number and randomly sampled. Twelve thousand records were selected. Data on patient sex and age, diagnosis, admission date, time laboratory tests were ordered, and the laboratory tests and their results were anonymously extracted from the records.
According to the standard guideline [21], data on blood culture were extracted when the cultures were performed from a set of two or three blood samples obtained simultaneously from different venipuncture sites for each order. Positive results were defined as at least one blood sample with non-commensal bacteria or a mixed growth of two bacteria with predominant species. Negative results were defined when there was no growth after incubation for 5 days. Contamination was defined when a commensal organism or mixed microbial flora with no predominant organism was detected. Positive urine culture results were defined as growth of ≥105 colony forming units per milliliter (CFU/mL) of a single microorganism or a mixed growth of two bacteria with predominant species in similar proportions at ≥105 CFU/mL. Negative results were defined as no growth after incubation for 2 days. Contamination was defined as growth of ≥105 CFU/mL of a commensal organism, mixed growth of microbial flora with no predominant organism, or growth of <105 CFU/mL except growth of the same bacteria as the consecutive previous positive one, which was defined as positive.
Statistical analyses were performed using Microsoft Excel 2019 version 16.0.6742.2048. Intergroup comparisons were made using the chi-square (χ2) test. Statistical significance was defined as p<0.01.
Results
From 12 000 medical records, there were 6734 female and 5266 male patients, aged between a few months to 102 years with an average age of 52.08 years (SD=21.29). Data on blood and urine cultures were extracted from 1052 and 510 records; the average ages of these patients were 60.46 years (SD=19.69) and 59.52 years (SD=23.23), respectively. Other patient characteristics are presented in Table 1.
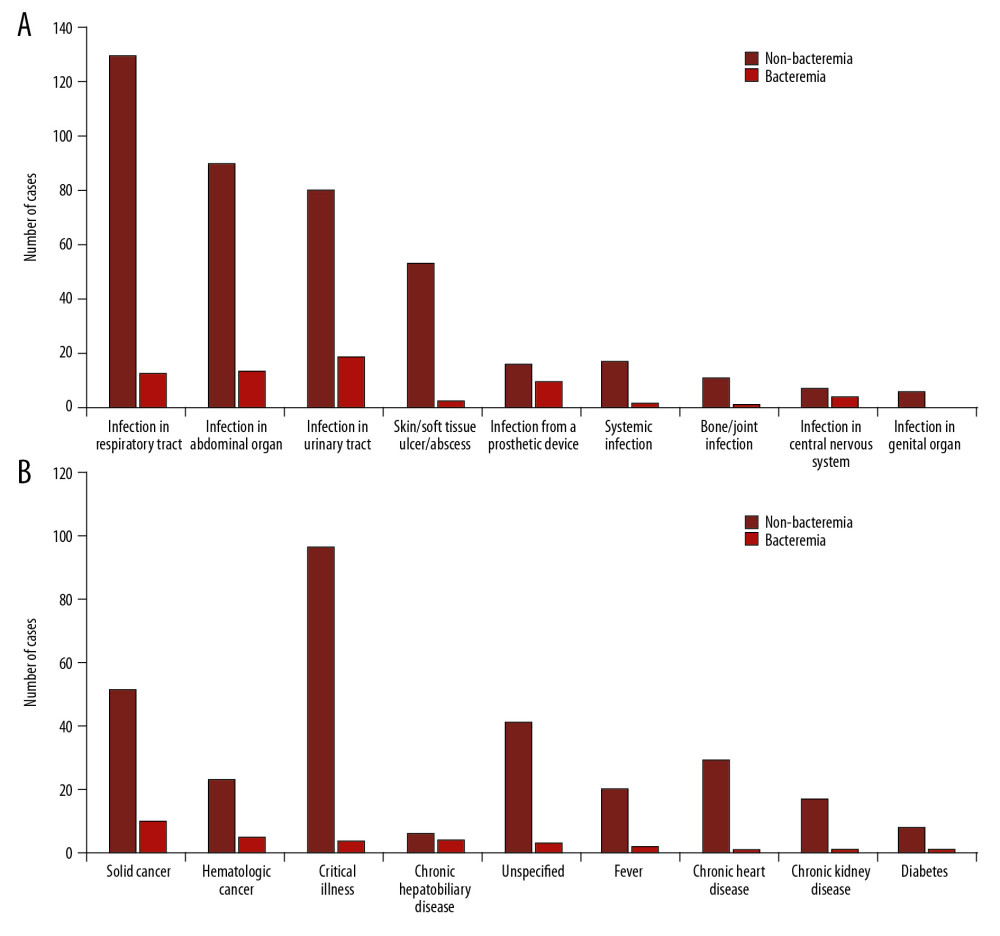
Sepsis was suspected in 13 cases and community-onset bacteremia was detected in nine of them (69.23%). Culture was also obtained within 48 hr after admission in 898 cases with other diagnoses; bacteremia was found in 97 of them; regarding the diagnoses, bacteremia was detected in small proportions of patients with each diagnosis (Figure 1). The first episode of blood culture was obtained after admission over 48 h in 141 cases; 19 of them had hospital-acquired bacteremia.
When multiple episodes of blood cultures were reviewed as independent events, there were 1041 blood sample sets obtained for culture within 48 h after admission and 876 sets obtained after admission over 48 h. Most of the results (1677 sample sets, 87.48%) were negative. Contamination was detected in 44 sample sets (2.30%). Community-onset and hospital-acquired bacteremia were found in 117 and 79 sample sets, respectively; in total, 10.22% were positive. Identified bacteria are listed in Table 2. Rates of community-onset and hospital-acquired bacteremia were 11.24% and 9.02%, respectively, which were non-significantly different [χ2=2.556, p=0.1099]. As shown in Table 3, there was a statistically significant association between blood culture results and percent of neutrophils.
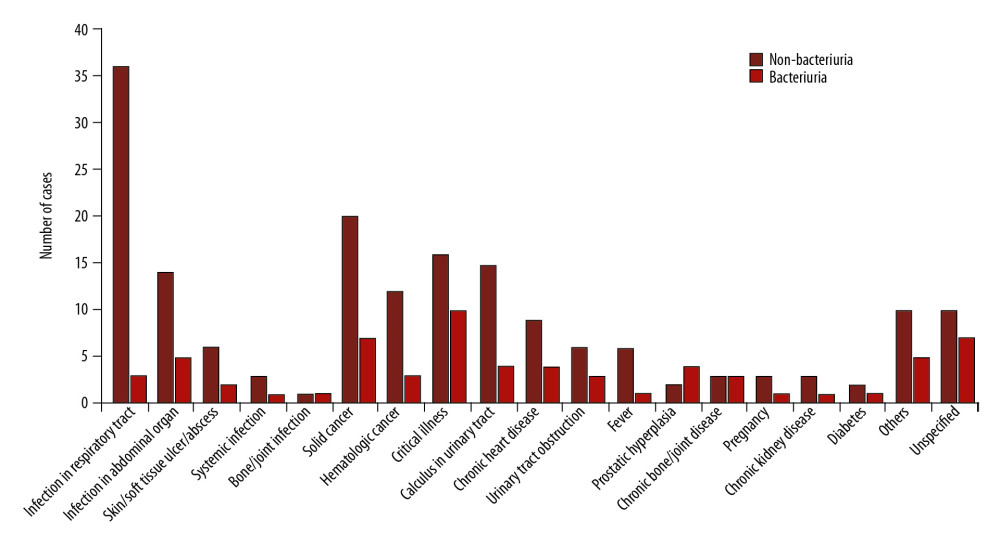
Urine samples were obtained for culture within 48 h after admission in 405 cases. UTI was suspected in 135 of them and 61 of these cases (45.19%) had bacteriuria. UTI was sought as a source of sepsis in six cases; bacteriuria was detected in one of them (16.67%). Community-onset bacteriuria was also detected in 66 cases of other diagnoses, as shown in Figure 2. The first urine sample was collected for culture after admission over 48 h in 105 cases; 17 of them had hospital-acquired bacteriuria.
A total 422 urine samples were obtained for culture within 48 h after admission and 301 were collected after admission over 48 h. No growth was totally reported in 299 cases (41.36%). Community-onset bacteriuria was detected in 135 samples and hospital acquired bacteriuria was detected in 63 samples, leading to a total positive rate of 27.38%; identified microorganisms are listed in Table 4. The rate of community-onset bacteriuria (31.99%) was significantly higher than the rate of hospital-acquired bacteriuria (20.93%) with the χ2 of 10.808 and p=0.0005.
As shown in Table 5, the rate of contamination of midstream voided urine was significantly higher than that for catheterized urine. In total, contamination was detected in 226 cases (31.26%). These patients had an average age of 59 years (mode=80 years). Results of urine nitrite and LE were significantly associated with results of urine culture (Table 6).
Discussion
As a tropical country, Thailand is expected to have a high incidence of infectious diseases including sepsis and UTI, but these two conditions were not often diagnosed in this community. Blood and urine cultures were used broadly, thus the limitations of a culture-based method did not restrict their use. Blood culture was used to support sepsis diagnosis in only 13 cases and the remaining cases, it was used to screen for sepsis in other illnesses, mainly respiratory tract infections (Figure 1). Respiratory tract infection has been reported to be the most common site of infection leading to sepsis in other regions [22–24] but it was not in this community, which might be a result of differences in regional and community health factors. Bacteremia was infrequently detected in these cases, but it was notably detected in cases of some local infections, cancers, and chronic hepatobiliary disease (Figure 1). In chronic hepatobiliary diseases, weakness of liver cells and disability of their functioning, specifically synthesis of immune-mediated cytokines and impairment of bile secretion, can increase risk of ascending bacterial invasion from the intestine [25,26]. According to previous reports [12,27,28], cancer is one of the most frequent preexisting comorbidities leading to bacteremia, particularly in patients receiving long-term immunosuppressive therapy.
Blood culture was repeatedly used in patients hospitalized for long periods. According to a previous report, risk of contracting bacteremia and subsequently having a blood culture taken increases with length of hospital stay [29]. Sepsis due to intravascular device or hospital-acquired infection reportedly is associated with the highest number of positive blood cultures [30], but some reports show that in most cases, sepsis was present on admission or had community onset [22,31]. In this Thai community, there was no significant difference between the rate of community-onset and hospital-acquired bacteremia.
The positive rate of blood culture was 10.22%, which was similar to that in previous reports [2,32,33]. Only one bacterium was identified in almost all positive cases, which is in accord with a previous report [11]. The most common cause of community-onset bacteremia was
As shown in Table 3, there were nonsignificant associations between blood culture results and both the number of white blood cells and serum lactate concentrations. As previously reported, leukocytosis alone is a poor predictor of bacteremia and not an indication for obtaining blood cultures [34]. Likewise, increased serum lactate levels represent tissue hypoperfusion associated with signs of organ dysfunction in various conditions, including sepsis [5,35]. There was a significant association between blood culture results and the percent of neutrophils, however, as presented in Table 3, bacteremia was also detected in 16 cases without neutrophilia. This should not occur in a life-threatening condition such as sepsis.
Although urine culture is indicated to support diagnosis of UTI, it was also used to screen for UTI in cases of other illness. As shown in Figure 2, detection of community-onset bacteriuria was common in cases of UTI as well as in cases of prostatic hyperplasia, chronic bone/joint disease, critical illness, and unspecified diagnosis. When patients’ bones and joints do not work properly, they may delay urination, leaving themselves vulnerable to UTI. The high rate of bacteriuria in cases with unspecified diagnoses may be related to patient age because most patients in this group were elderly with an average age of 60 years (mode=78 years). As previously reported, clinical presentations of UTI in elderly patients are difficult to assess due to impaired communication and the frequent presence of chronic symptoms [36,37].
Urine culture had a high rate of contamination, which often led to repetitive urine culture. Contamination rates were significantly higher in cultures of midstream voided urine than in catheterized urine, in accord with a previous report [38]. This finding may also be related to patient age because 52% of these cases were aged over 60 years. Most elderly patients have physical impairments that cause inherent difficulty in self-urine collection, therefore, more invasive collection methods are required to establish a reliable diagnosis [20,38–40].
The most common causative bacterium in both community onset and hospital acquired bacteriuria was
As seen in Table 6, results of testing for LE and nitrite were significantly associated with bacteriuria. These findings support a previous suggestion that when LE or nitrite are negative, the likelihood is that a urine culture obtained from the patient will also be negative, and urine culture can be restricted to reduce cost and the number of specimens that need to be cultured in a tedious and time-consuming procedure [18,19].
Conclusions
A high negative rate of blood culture may be a result not only of the test’s low sensitivity but also of test use to screen for sepsis in various illnesses. Bacteremia is often detected in patients with certain local infections, chronic hepatobiliary disease, and cancers. Urine cultures can be used more appropriately if samples are collected properly. Besides UTI, bacteriuria is often found in cases with urinary tract obstruction, particularly prostatic hyperplasia, chronic bone and joint diseases, critical illness, and unspecified illnesses.
Tables
Table 1. Patient characteristics.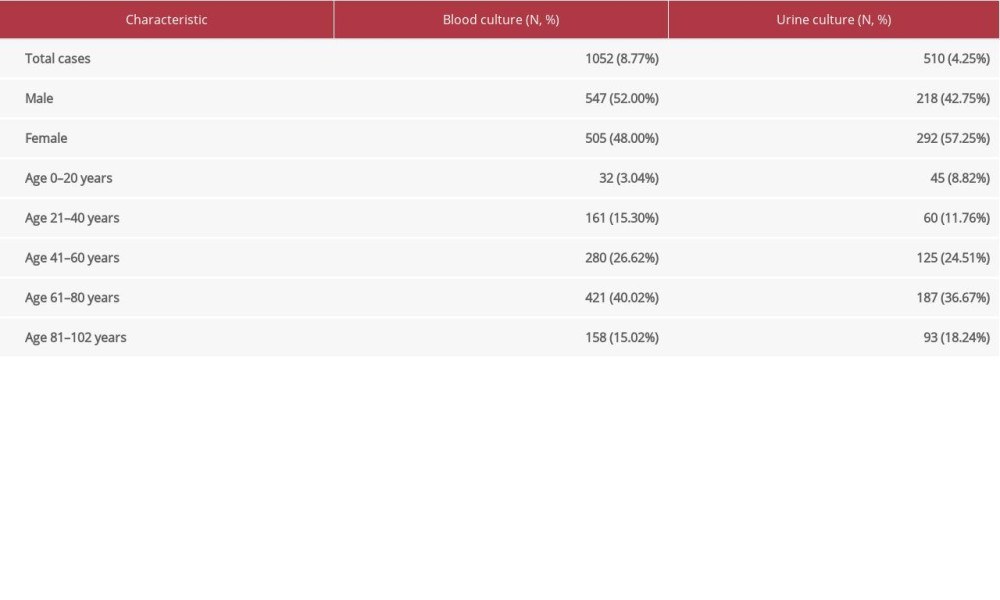 Table 2. Bacteria identified in community-onset and hospital-acquired bacteremia.
Table 2. Bacteria identified in community-onset and hospital-acquired bacteremia.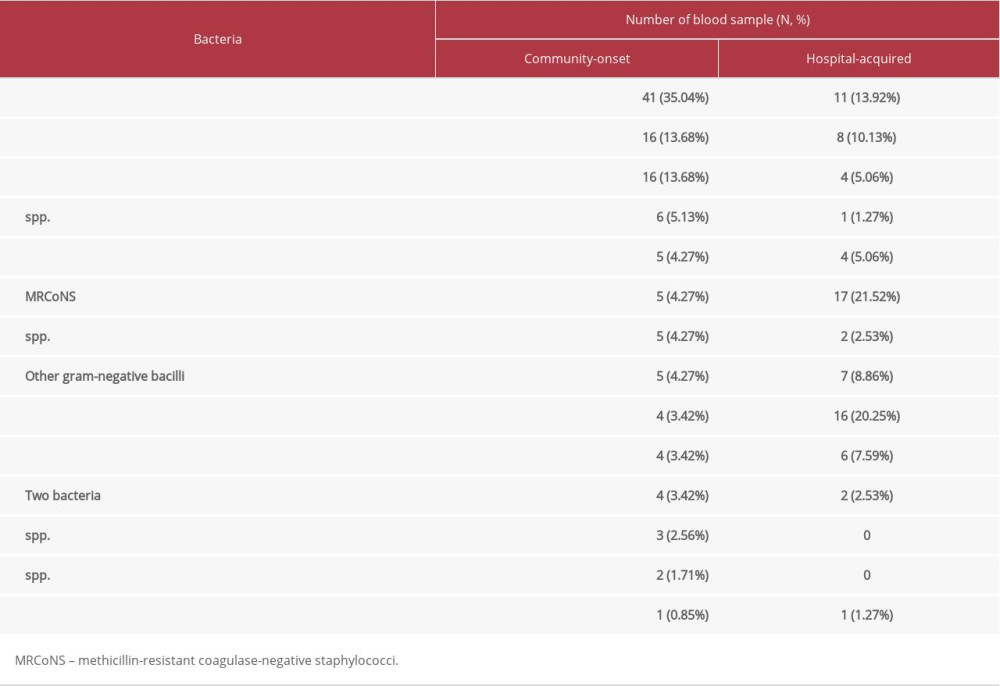 Table 3. Associations between results of blood culture and other related tests.
Table 3. Associations between results of blood culture and other related tests.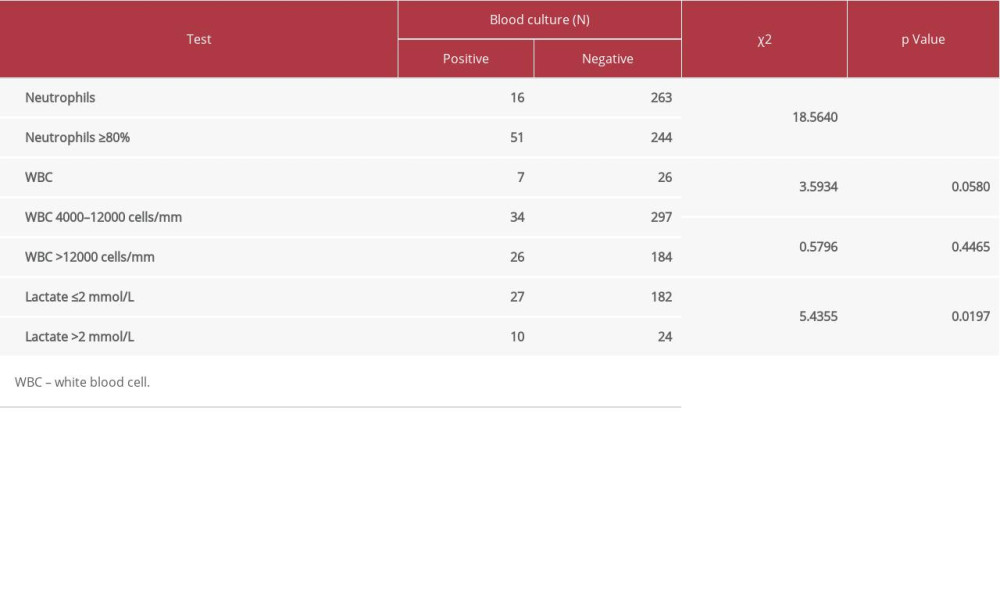 Table 4. Microorganism identified in community-onset and hospital-acquired bacteriuria.
Table 4. Microorganism identified in community-onset and hospital-acquired bacteriuria.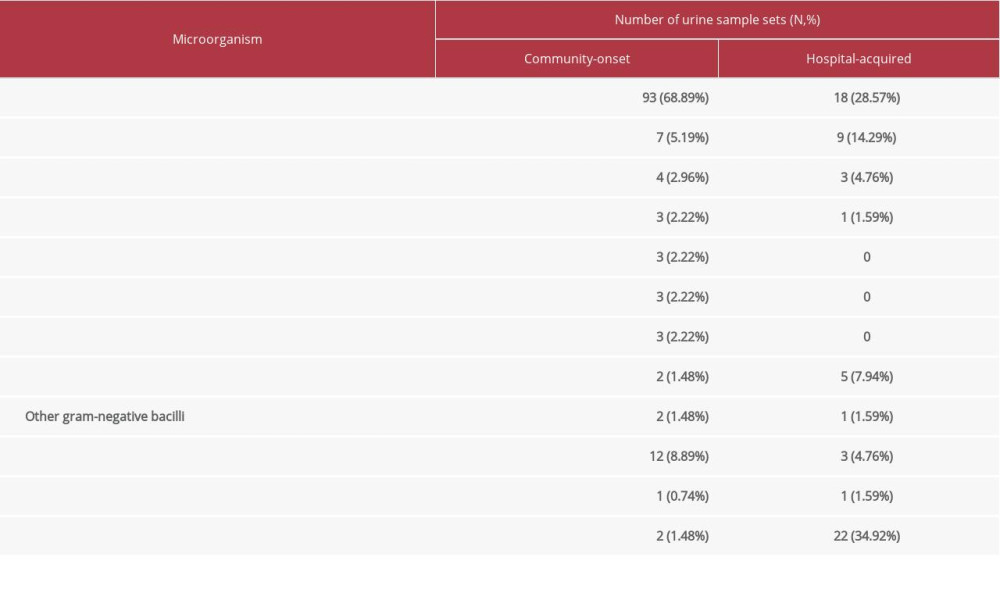 Table 5. Yield of cultures of catheterized and midstream voided urine.
Table 5. Yield of cultures of catheterized and midstream voided urine.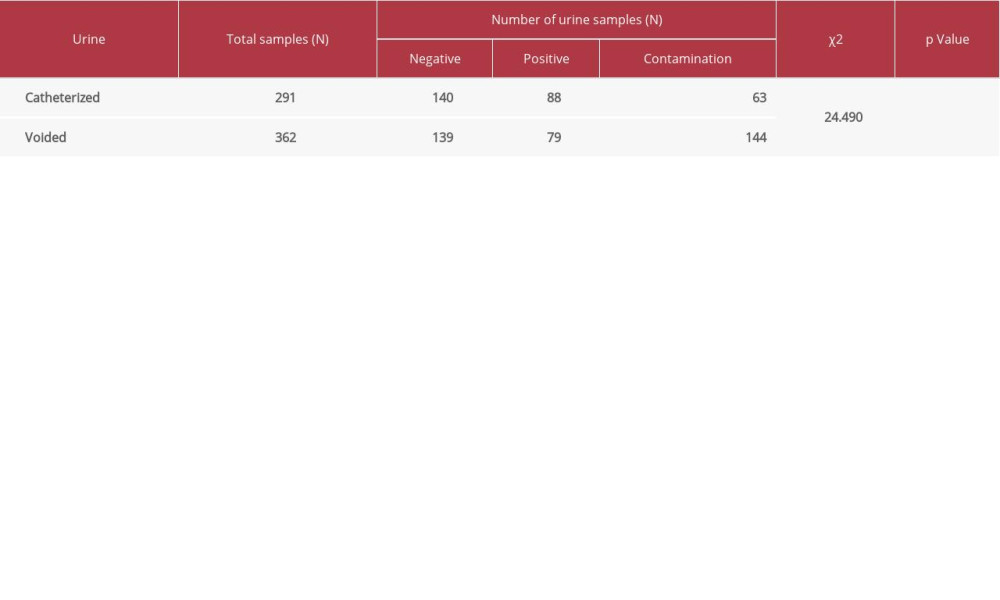 Table 6. Associations between results of nitrite/leucocyte esterase (LE) and urine culture.
Table 6. Associations between results of nitrite/leucocyte esterase (LE) and urine culture.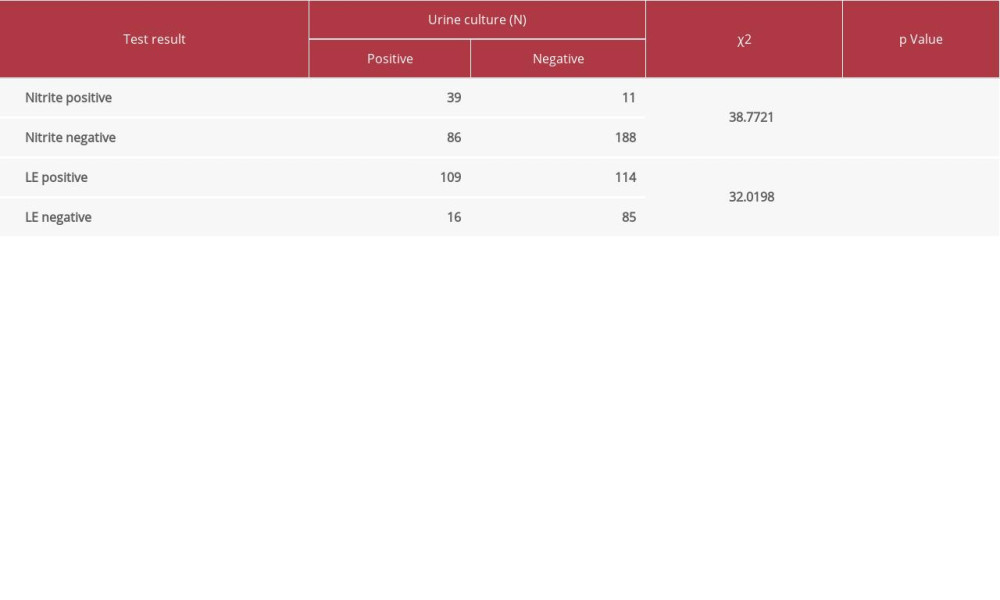
References
1. Long B, Koyfman A, Best clinical practice: Blood culture utility in the emergency department: J Emerg Med, 2016; 51(5); 529-39
2. Snyder JW, Blood cultures: The importance of meeting pre-analytical requirements in reducing contamination, optimizing sensitivity of detection, and clinical relevance: Clin Microbiol Newsl, 2015; 37(7); 53-57
3. Rello J, van Engelen TSR, Alp E, Towards precision medicine in sepsis: A position paper from the European Society of Clinical Microbiology and Infectious Diseases: Clin Microbiol Infect, 2018; 24; 1264-72
4. Coburn B, Morris AM, Tomlinson G, Detsky AS, Does this adult patient with suspected bacteremia require blood cultures?: JAMA, 2012; 308; 502-11
5. Riedel S, Predicting bacterial versus viral infection, or none of the above current and future prospects of biomarkers: Clin Lab Med, 2019; 39; 453-72
6. Rhodes A, Evans LE, Alhazzani W, Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016: Intensive Care Med, 2017; 43; 304-77
7. Makic MBF, Bridges E, Managing sepsis and septic shock: Current guidelines and definitions. Recent updates emphasize early recognition and prompt intervention: Am J Nurs, 2018; 118(2); 34-39
8. Dellinger RP, Levy MM, Rhodes A, Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012: Intensive Care Med, 2013; 39; 165-228
9. Lin W, Huang Y, Wang J: BMC Infect Dis, 2019; 19; 245
10. Hernández C, Fehér C, Soriano A, Clinical characteristics and outcome of elderly patients with community-onset bacteremia: J Infect, 2015; 70; 135-43
11. Ombelet S, Barbé B, Affolabi D, Best practices of blood cultures in low- and middle-income countries: Front Med, 2019; 6; 131
12. Khan HA, Baig FK, Mehboob R, Nosocomial infections: Epidemiology, prevention, control and surveillance: Asian Pac J Trop Biomed, 2017; 7(5); 478-82
13. Martinez RM, Wolk DM, Bloodstream infections: Microbiol Spectrum, 2016; 4(4) DMIH2-0031-2016
14. Hooton TM, Roberts PL, Cox ME, Stapleton AE, Voided midstream urine culture and acute cystitis in premenopausal women: N Engl J Med, 2013; 369; 1883-91
15. Foxman B, The epidemiology of urinary tract infection: Nat Rev Urol, 2010; 7; 653-60
16. Oyaert M, Meensel BV, Cartuyvels R, Laboratory diagnosis of urinary tract infections: Towards a BILULU consensus guideline: J Microbiol Methods, 2018; 146; 92-99
17. Pezzlo M, Laboratory diagnosis of urinary tract infections: Guidelines, challenges, and innovations: Clin Microbiol Newsl, 2014; 36(12); 87-93
18. Garcia R, Spitzer ED, Promoting appropriate urine culture management to improve health care outcomes and the accuracy of catheter-associated urinary tract infections: Am J Infect Control, 2017; 45; 1143-53
19. Marques AG, Doi AM, Pasternak J, Performance of the dipstick screening test as a predictor of negative urine culture: Einstein, 2017; 15(1); 34-39
20. Foxman B, Urinary tract infection syndromes occurrence, recurrence, bacteriology, risk factors, and disease burden: Infect Dis Clin N Am, 2014; 28; 1-13
21. Wilson ML, Mitchell M, Morris AJ: Principles and procedures for blood cultures, 2007, Wayne. Pennsylvania, Clinical and Laboratory Standards Institute (CLSI)
22. Cecconi M, Evans L, Levy M, Rhodes A, Sepsis and septic shock: Lancet, 2018; 392; 75-87
23. Martin GS, Sepsis, severe sepsis and septic shock: Changes in incidence, pathogens and outcomes: Expert Rev Anti Infect Ther, 2012; 10(6); 701-6
24. Ljungström L, Andersson R, Jacobsson G, Incidences of community onset severe sepsis, Sepsis-3 sepsis, and bacteremia in Sweden – A prospective population-based study: PLoS One, 2019; 14(12); e0225700
25. Geier A, Fickert P, Trauner M, Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis: Nat Rev Gastroenterol Hepatol, 2006; 3; 574-85
26. Strnad P, Tacke F, Koch A, Liver – guardian, modifier and target of sepsis: Nat Rev Gastroenterol Hepatol, 2017; 14; 55-66
27. Rosolem MM, Rabello LSCF, Lisboa T, Critically ill patients with cancer and sepsis: Clinical course and prognostic factors: J Crit Care, 2012; 27(3); 301-7
28. Husak L, Marcuzzi A, Herring J, National analysis of sepsis hospitalizations and factors contributing to sepsis in-hospital mortality in Canada: Healthc Q, 2010; 13; 35-41
29. Gubbels S, Nielsen J, Voldstedlund M, Utilization of blood cultures in Danish hospitals: A population-based descriptive analysis: Clin Microbiol Infect, 2015; 21(4); 344.e13-21
30. Jeganathan N, Yau S, Ahuja N, The characteristics and impact of source of infection on sepsis-related ICU outcomes: J Crit Care, 2017; 41; 170-76
31. Laupland KB, Incidence of bloodstream infection: A review of population-based studies: Clin Microbiol Infect, 2013; 19; 492-500
32. Miller JM, Binnicker MJ, Campbell S, A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology: Clin Infect Dis, 2018; 67(6); e1-94
33. Khatib R, Simeunovic G, Sharma M, Blood culture series benefit may be limited to selected clinical conditions: Time to reassess: Clin Microbiol Infect, 2015; 21; 332-36
34. Riley LK, Rupert J, Evaluation of patients with leukocytosis: Am Fam Physician, 2015; 92(11); 1004-11
35. Lee SM, An WS, New clinical criteria for septic shock: Serum lactate level as new emerging vital sign: J Thorac Dis, 2016; 8(7); 1388-90
36. Nicolle LE, Urinary tract infections in the older adult: Clin Geriatr Med, 2016; 32; 523-38
37. Froom P, Shimoni Z, The uncertainties of the diagnosis and treatment of a suspected urinary tract infection in elderly hospitalized patients: Expert Rev Anti Infect Ther, 2018; 16(10); 763-70
38. Kubik MJ, McCarter YS, Controversies in the diagnosis of urinary tract infections: Clin Microbiol Newsl, 2012; 34(23); 185-91
39. Wilson ML, Gaido L, Laboratory diagnosis of urinary tract infections in adult patients: Clin Infect Dis, 2004; 38; 1150-58
40. Pescatore R, Niforatos JD, Rezaie S, Swaminathan A, Evidence-informed practice: diagnostic questions in urinary tract infections in the elderly: West J Emerg Med, 2019; 20(4); 573-77
41. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ, Urinary tract infections: Epidemiology, mechanisms of infection and treatment options: Nat Rev Microbiol, 2015; 13(5); 269-84
42. Price TK, Dune T, Hilt EE, The clinical urine culture: Enhanced techniques improve detection of clinically relevant microorganisms: J Clin Microbiol, 2016; 54(5); 1216-22
43. Kauffman CA, Fisher JF, Sobel JD, Newman CA, Candida urinary tract infections – diagnosis: Clin Infect Dis, 2011; 52(Suppl 6); S452-56
Figures
Tables
 Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Bacteria identified in community-onset and hospital-acquired bacteremia.
Table 2. Bacteria identified in community-onset and hospital-acquired bacteremia. Table 3. Associations between results of blood culture and other related tests.
Table 3. Associations between results of blood culture and other related tests. Table 4. Microorganism identified in community-onset and hospital-acquired bacteriuria.
Table 4. Microorganism identified in community-onset and hospital-acquired bacteriuria. Table 5. Yield of cultures of catheterized and midstream voided urine.
Table 5. Yield of cultures of catheterized and midstream voided urine. Table 6. Associations between results of nitrite/leucocyte esterase (LE) and urine culture.
Table 6. Associations between results of nitrite/leucocyte esterase (LE) and urine culture. Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Bacteria identified in community-onset and hospital-acquired bacteremia.
Table 2. Bacteria identified in community-onset and hospital-acquired bacteremia. Table 3. Associations between results of blood culture and other related tests.
Table 3. Associations between results of blood culture and other related tests. Table 4. Microorganism identified in community-onset and hospital-acquired bacteriuria.
Table 4. Microorganism identified in community-onset and hospital-acquired bacteriuria. Table 5. Yield of cultures of catheterized and midstream voided urine.
Table 5. Yield of cultures of catheterized and midstream voided urine. Table 6. Associations between results of nitrite/leucocyte esterase (LE) and urine culture.
Table 6. Associations between results of nitrite/leucocyte esterase (LE) and urine culture. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176