26 May 2021: Laboratory Research
Cytotoxicity and Antiplasmodial Properties of Different Peel Extracts
Rudi Hendra12ABCDEFG*, Rohimatul Khodijah1B, Rianti Putri3BC, Riezki Amalia24BC, Yuli Haryani1DF, Hilwan Yuda Teruna1DEF, Rizky Abdulah24DEFDOI: 10.12659/MSMBR.931118
Med Sci Monit Basic Res 2021; 27:e931118
Abstract
BACKGROUND: Dragon fruit (Hylocereus polyrhizus) is one of the most common fruits in tropical countries, including Indonesia. The unique deep purple-colored pulp of the fruit is eaten whole and consumed as juice. However, the inedible thick peel is wasted, causing environmental issues. In this study, the toxic, cytotoxic, and antiplasmodium activity from various extract of H. polyrhizus peels were examined.
MATERIAL AND METHODS: We evaluated the cytotoxicity and antiplasmodial properties of the various peel extracts by using different organic solvents.The extraction of the peels was conducted using maceration to obtain pigment, n-hexane, dichloromethane, and ethyl acetate extracts. The toxicity of the extract was assessed using the brine shrimp lethality test, followed by WST assay to test in vitro cytotoxic properties and in vitro antiplasmodial properties in 2 Plasmodium falciparum strains (3D7 and W2).
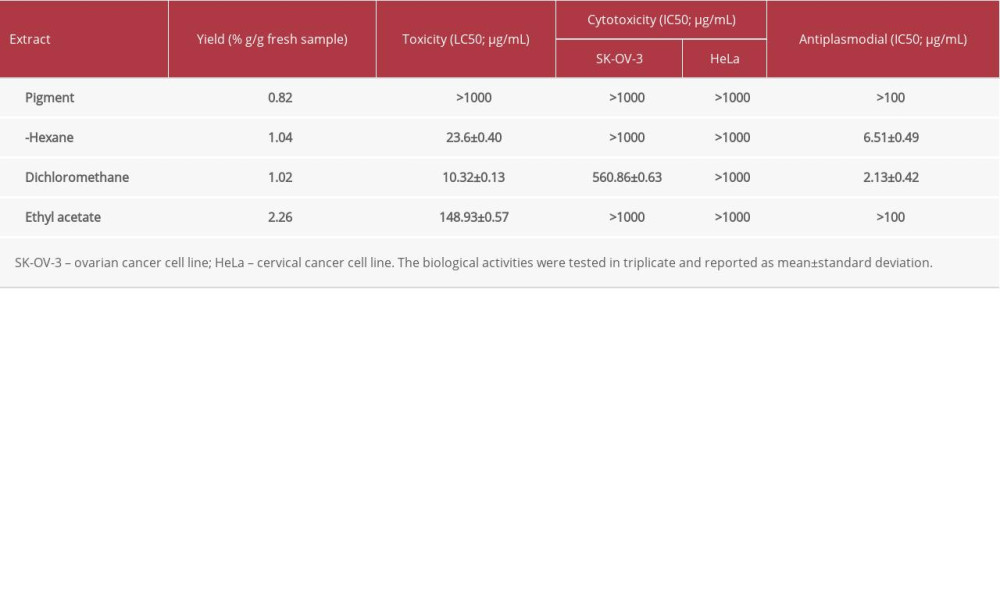
RESULTS: The n-hexane, dichloromethane, and ethyl acetate extracts depicted various levels of activity, whereas the pigment extract did not show any activities. However, dichloromethane demonstrated a high toxicity level with LC₅₀ of 10.32±0.13 μg/mL and a weak cytotoxic level against SK-OV-3 cell lines (IC₅₀ of 560.86±0.63 μg/mL). Moreover, the dichloromethane and n-hexane extracts showed high and promising antiplasmodial activity with IC₅₀ 2.13±0.42 and 6.51±0.49 μg/mL, respectively.
CONCLUSIONS: The dichloromethane extract demonstrated high antiplasmodial activity. Our observations have elucidated the cytotoxic and antiplasmodial activity of the peel of dragon fruits and can be used as a foundation for further research into the isolation and bioactivity of secondary metabolites.
Keywords: Antimalarials, Cytotoxicity Tests, Immunologic, Plant Extracts, Toxicity Tests, Cactaceae, Fruit, Methylene Chloride, Plasmodium falciparum
Background
Dragon fruit’s exotic aesthetic appearance, with its enticing deep purple-colored pulp, makes the fruit highly appealing in the European, U.S., and Asian markets. The deep purple color of the pulp comes from a group of pigments known as nitrogen-containing betalains [5]. Wybraniec et al fully characterized 3 major betalains along with 7 minor betalains present in the pulp and peel of
Despite the unique red color and shape of this plant, it is one of the most well-known fruits in tropical countries, including Indonesia, where it is consumed in beverages and for dessert. The thickness of the peel (22% of its total weight) causes environmental problems with waste since it is not used optimally. The pigment extract from this fruit peel demonstrated good free radical-scavenging activity by DPPH assay in our previous study, with 159.6 μg/mL of half-maximal inhibitory concentration (IC50) [9]. Apart from its antioxidant properties, Vijayakumar et al reported that
Moreover, the pigment and ethyl acetate extracts inhibited 60% to 75% of growth for
Material and Method
EXTRACTION:
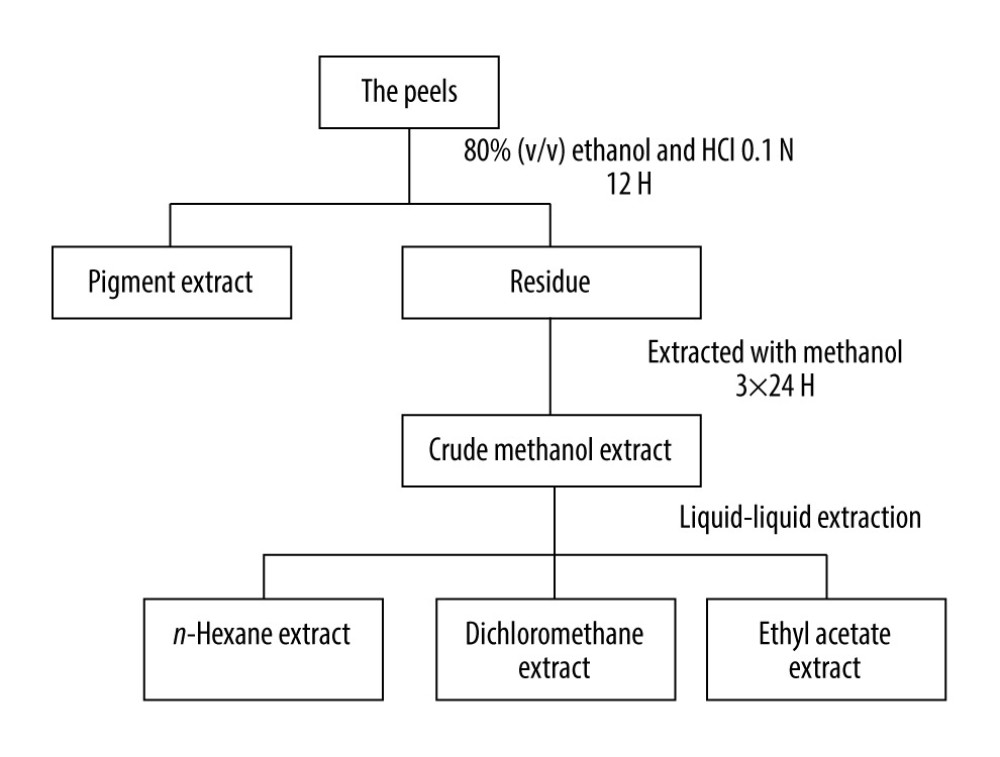
Dragon fruit was obtained from a traditional market in Pekanbaru City, Indonesia. The fresh peels were cleaned, cut into small pieces, blended, and kept in the refrigerator until use. The extraction was divided into 2 steps, pigment and non-pigment extractions, as reported by Hendra et al (Figure 1) [9,12,13]. The extraction was conducted using the organic solvents ethanol, n-hexane, dichloromethane, and ethyl acetate.
TOXICITY ASSAY:
The level of toxicity of the extracts was calculated using the brine shrimp lethality test (BSLT). The test method was performed by preparing 10 vials, each filled with 2 mL of seawater, and a 2-fold dilution was produced to generate a number of concentrations. An aliquot (0.1 mL) with approximately 10 nauplii were added to each vial and allowed to sit for 24 h. The vials were observed and dead larvae were counted after 24 h. Dimethyl sulfoxide (DMSO) was used as a negative control. Based on the mortality percentage, the concentration that led to 50% mortality (LC50) was determined by the use of the graph of median mortality percentage vs log of concentration [14,15]. The assay was done in triplicate, and the data were reported as mean±standard deviation.
CYTOTOXICITY (MTS) ASSAY:
Ovarian cancer cell line (SK-OV-3) and cervical cancer cell line (HeLa) cells were obtained from the collection of the Department of Pharmacology and Clinical Pharmacy, Universitas Padjadjaran. The cells were grown in Dulbecco’s modified Eagle’s medium (Gibco, Paisley, UK) containing 10% of heat-inactivated fetal bovine serum (Gibco, Paisley, UK) and 1% penicillin-streptomycin solution (Gibco, Paisley, UK). The cytotoxicity assay was performed in 96-well plates, and cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. Cells with 70% to 80% confluency were refreshed with new serum-free medium, further incubated for 4 h, and then treated with extracts in the amounts of 7.8125 μg/mL 15.625 μg/mL, 31.25 μg/mL, 62.5 μg/mL, 125 μg/mL, 250 μg/mL, 500 μg/mL, and 1000 μg/mL. After 24 h of incubation, reagent cell counting kit-8 (Dojindo, Rockville, MD, USA) was added, and the mixtures were incubated for 2 h. DMSO was used as a negative control to avoid false results and the standard drug cisplatin was used as a positive control. The absorbance of the cell suspensions was measured using a Tecan Infinite spectrophotometer (Tecan, Grodig, Austria) at 450 nm [16]. The assay was done in triplicate and the data were reported as mean±standard deviation.
:
Chloroquine-sensitive 3D7 and chloroquine-resistant W2
Based on the percentage of inhibition results, statistical analysis was performed with a probit analysis using SPSS version 20 to determine the IC50 value or concentration of the test material that could inhibit the growth of the parasite by as much as 50% [17]. The assay was done in triplicate and the data were reported as mean ± standard deviation.
STATISTICAL ANALYSIS:
SPSS version 20 was used for all analyses. Assays were performed in triplicate and data were reported as mean±standard deviation.
Results
In this present study, the fresh peels of
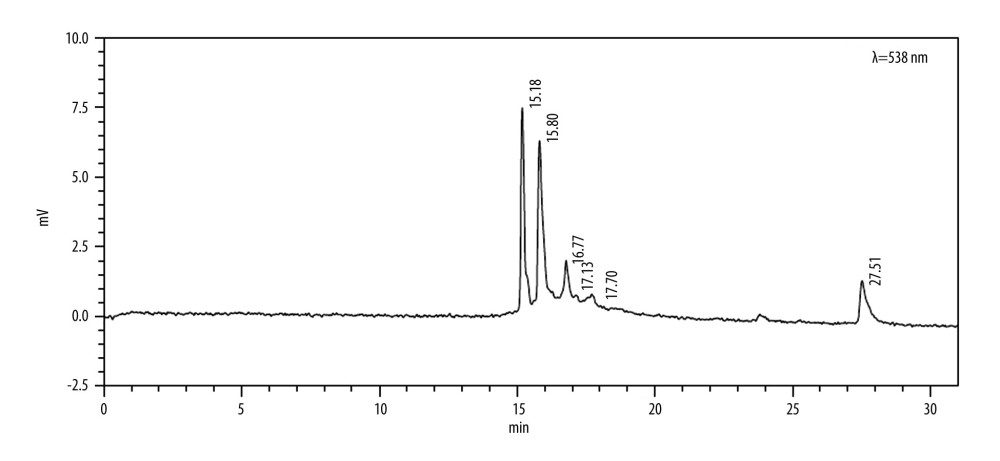
To investigate the pigment compounds in the extract, the extracts were analyzed using high-performance liquid chromatography (HPLC). The results are shown in Figure 2.
Discussion
In this present study, the fresh peels of
The toxicity levels were measured using the BSLT, a simple, high-performance toxicity test for bioactive substances or extracts that is based on the ability of test compounds to kill shrimp (
After the BSLT study, the potency of the extracts as anticancer properties was determined and the cytotoxic activity in SK-OV-3 and HeLa cell lines was examined using the MTS assay. Studies have shown that the in vitro cytotoxicity MTS assay is a practical way to evaluate cell viability. The key features of this test are its ease of use, precision, and rapid toxicity result. The MTS assay may also be a valuable method in assessing risk to human health if adequate sensitivity and specificity of the test are demonstrated [23]. Table 1 shows the various cytotoxicity levels of the extracts in SK-OV-3 and HeLa cell lines. None of the extracts showed toxicity against HeLa cell lines. However, the extracts of dichloromethane showed weak toxicity to SK-OV-3, with an IC50 value of 560.86±0.63 μg/mL; this finding is in agreement with that of the U.S. National Cancer Institute [24]. In the present study, the peel extracts showed weak activity against both cancer cell lines. However, Luo et al reported that the peel extracts inhibited different cancer cells (PC3, Bcap-37, and MGC-803 cell lines), with IC50 values ranging from 450 μg/mL to 500 μg/mL [8]. Surprisingly, the pigment (betalain) extract did not display cytotoxicity toward either cell line. However, in another study, betalain extracts from cactus pear fruit (
In the present study, all 4 extracts were found to have various levels of activity against chloroquine-sensitive 3D7 and chloroquine-resistant W2 strains of
Regarding the chemical composition of the peel of this species, Lou et al (2018) identified 2 major phytosterols, β-amyrin and β-sitosterol [8], which could be responsible for the cytotoxicity and antiplasmodial activity of the extract. Isolated β-amyrin from
Conclusions
This research has shown the promising cytotoxicity and antiplasmodial activity of
References
1. Wybraniec S, Platzner I, Geresh S: Phytochemistry, 2001; 58(8); 1209-12
2. Hernández YDO, Salazar JAC: Comun Sci, 2012; 3(4); 220-37
3. Wu L-C, Hsu H-W, Chen Y-C, Antioxidant and antiproliferative activities of red pitaya: Food Chem, 2006; 95(2); 319-27
4. Nurmahani M, Osman A, Hamid AA: Int Food Res J, 2012; 19(1); 77
5. Rebecca O, Boyce A, Chandran S: Afr J Biotechnol, 2010; 9(10); 1450-54
6. Wybraniec S, Nowak-Wydra B, Mitka K: Phytochemistry, 2007; 68(2); 251-59
7. Ariffin AA, Bakar J, Tan CP, Essential fatty acids of pitaya (dragon fruit) seed oil: Food Chem, 2009; 114(2); 561-64
8. Luo H, Cai Y, Peng Z, Chemical composition and in vitroevaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel: Chem Cent J, 2014; 8(1); 1
9. Hendra R, Masdeatresa L, Abdulah R, Haryani Y, AIP Publishing LLC
10. Vijayakumar R, Abd Gani SS, Zaidan UH: Evid Based Complement Alternat Med, 2020; 2020; 7520736
11. Azwanida N, Normasarah N, Afandi A: Jurnal Teknologi, 2014; 69(6); 3326
12. Hendra R, Masdeatresa L, Abdulah R, Haryani Y: J Physic, IOP Publishing
13. Hendra R, Masdeatresa L, Almurdani M
14. Jasril J, Teruna HY, Aisyah A, Nurlaili N, Hendra R, Microwave assisted synthesis and evaluation of toxicity and antioxidant activity of pyrazoline derivatives: Indones J Chem, 2019; 19(3); 583-91
15. Syahmi ARM, Vijayarathna S, Sasidharan S: Molecules, 2010; 15(11); 8111-21
16. Rachmawati H, Sundari S, Nabila N: Biochem Biophys Res Commun, 2019; 515(1); 99-103
17. Jansen O, Tits M, Angenot L: Malar J, 2012; 11(1); 289
18. Strack D, Vogt T, Schliemann W, Recent advances in betalain research: Phytochemistry, 2003; 62(3); 247-69
19. Liang Y-Z, Xie P, Chan K, Quality control of herbal medicines: J Chromatogr B, 2004; 812(1–2); 53-70
20. Wu C, An important player in brine shrimp lethality bioassay: The solvent: J Adv Pharm Technol Res, 2014; 5(1); 57
21. Meyer B, Ferrigni N, Putnam J, Brine shrimp: A convenient general bioassay for active plant constituents: Planta Med, 1982; 45(1); 31-34
22. Ghisalberti EL, Detection and isolation of bioactive natural products: Bioactive natural products: Detection, isolation, and structural determination, 2008; 18, Florida, CRC Press
23. Malich G, Markovic B, Winder C, The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines: Toxicology, 1997; 124(3); 179-92
24. Widiyastuti Y, Sholikhah IYM, Haryanti S, 2019; 2202(1), AIP Publishing LLC, doi: 10.1063/1-5141714
25. Zou D, Brewer M, Garcia F, Cactus pear: A natural product in cancer chemoprevention: Nutr J, 2005; 4(1); 1-12
26. Fidock DA, Rosenthal PJ, Croft SL, Antimalarial drug discovery: Efficacy models for compound screening: Nat Rev Drug Discov, 2004; 3(6); 509-20
27. Chung IM, Kim MY, Park SD: Phytother Res, 2009; 23(11); 1634-37
28. Kim KH, Choi SU, Kim CS, Lee KR: Biosci Biotechnol Biochem, 2012; 76(4); 825-27
29. Mwangi E, Keriko J, Machocho A: J Med Plants Res, 2010; 4(9); 726-31
Figures
Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176