05 May 2022: Laboratory Research
Calcitriol Inhibits Proliferation and Potentially Induces Apoptosis in B16–F10 Cells
Eva Krishna Sutedja1ABDEF, Daniar Amarassaphira2ABF, Hanna Goenawan34BCEF, Yuni Susanti Pratiwi34BDE, Nova Sylviana34BDF, Budi Setiabudiawan5ABDEF, Oki Suwarsa1BDEF, Raden Tina Dewi Judistiani6CEF, Unang Supratman7BEF, Ronny Lesmana34ABCDEFG*DOI: 10.12659/MSMBR.935139
Med Sci Monit Basic Res 2022; 28:e935139
Abstract
BACKGROUND: Melanoma is one of the most aggressive types of cancer and it has shown a remarkable surge in incidence during the last 50 years. Melanoma has been projected to be continuously rising in the future. Therapy for advanced-type melanoma is still a challenge due to the low response rate and poor 10-year survival. Interestingly, several epidemiological and preclinical studies had reported that vitamin D deficiency was associated with disease progression in several cancer types. In vivo and in vitro studies revealed anti-proliferative, anti-angiogenic, apoptosis, and differentiation induction effects of calcitriol in various cancers. However, information on the effects of calcitriol (1,25(OH)₂D₃) on melanoma is still limited, and its mechanism remains unclear.
MATERIAL AND METHODS: In the present study, by utilizing B16-F10 cells, which is a melanoma cell line, we explored the anti-proliferative effect of calcitriol using cell viability assay, near-infrared imaging, expression of apoptosis-related genes using real-time polymerase chain reactions (PCR), and the expression of apoptosis proteins levels using western blot. In addition, we also assessed calcitriol uptake by B16-F10 cells using high-performance liquid chromatography (HPLC).
RESULTS: We found that calcitriol inhibits melanoma cell proliferation with an IC₅₀ of 93.88 ppm (0.24 μM), as shown by cell viability assay. Additionally, we showed that B16-F10 cells are capable of calcitriol uptake, with a peak uptake time at 60 min after administration. Calcitriol was also able to induce apoptosis-related proteins such as caspase-3, caspase 8, and caspase-9. These effects of calcitriol reflect its potential utility as a potent adjuvant therapy for melanoma.
CONCLUSIONS: Calcitriol inhibits cell proliferation and induces apoptosis in B16-F10 cells.
Keywords: Apoptosis, Calcitriol, Caspases, Melanoma, Experimental, Animals, Cell Line, Tumor, Cell Proliferation
Background
Its geographical proximity to the equator and tropical climate expose Indonesia to high levels of ultraviolet (UV) radiation throughout the year, which results in high risk of skin cancer, including melanoma [1,2]. Melanoma is a malignancy that emerges from an abnormal proliferation of melanocytes, which are pigment-producing cells. Although melanoma is historically quite rare, it is now the 19th most common cancer worldwide [3–5]. Compared to other skin cancers, the incidence of melanoma is 10 times higher than that of other skin cancers [3]. Fair-skinned white people and people living in low-latitude regions have been reported as high-risk populations [4].
Early detection and surgery can be the therapy of choice for localized tumors, but therapy for metastatic type melanoma is still ineffective [2,6]. Once its metastasizes, the 10-year survival rate is only about 10% [7]. Newer chemotherapy agents such as vemurafenib (BRAF kinase inhibitor) and trametinib (MEK kinase inhibitor) showed a better response rate, with vemurafenib approaching a 50% response rate after treatment. However, there is still a risk of treatment resistance with these specific kinase inhibitors, and combination therapy showed a significant improvement compared to single therapy [7,8]. Therefore, adjuvant therapies in melanoma with differing treatment approaches may help to significantly increase survival. Vitamin D is a promising adjunctive therapy, especially since there have been many investigations about the anti-cancer effects of vitamin D in recent decades [6,9,10].
Vitamin D is a fat-soluble prohormone that is synthesized mainly by the production of vitamin D3 or calcitriol by ultraviolet B (UVB) exposure of the skin (90%) and to a lesser extent is obtained from the diet (10%) in the form of vitamin D2 [11,12]. Vitamin D needs to be activated by 2 primary hydroxylation processes before eliciting numerous biological actions [13]. Both forms will bind vitamin D bound (VDB) in the bloodstream to undergo two-step hydroxylation by 25-hydroxylase in hepatocytes and 1α-hydroxylase in the kidney to produce its active form, calcitriol [11]. Vitamin D receptor (VDR) plays a crucial role in the genomic pathway. The binding of vitamin D and VDR forms a nuclear receptor-ligand complex [14]. VDR forms a dimer with retinoid X receptor (RXR) and translocates in the nucleus [15]. Ligands-bound VDR-RXR binds vitamin D response elements (VDREs) in a target gene promoter region that exerts autocrine and paracrine effects [9,12]. Alternative pathways of vitamin D activation in the body include another novel pathway of D3 metabolism that operates in vivo and is initiated and regulated by P450scc, modified by CYP27B1, and its products and intermediates are biologically active [16]. Vitamin D2 metabolism is initiated by CYP11A1 and modified by CYP27B1, with the product profile showing tissue- and cell-type specificity [17]. Products of CYP11A1 action on 7DHC, namely 22(OH)7DHC, 20,22(OH)27DHC, and 7-dehydropregnenolone, were also detected in serum, epidermis, and the adrenal. Thus, we have detected novel CYP11A1-derived secosteroids in the skin, serum and adrenal gland, and based on their concentrations and biological activity, suggest that they act as hormones in vivo [18]. As an alternative nuclear receptor for vitamin D, a study showed the protection of human keratinocytes against DNA damage included the activation of the NRF2-regulated antioxidant response, p53-phosphorylation and its translocation to the nucleus, and DNA repair induction. These data indicate that novel derivatives of vitamin D3 and lumisterol are promising photoprotective agents [19].
It was previously shown that calcitriol plays a vital role in an endocrine manner by regulating calcium and phosphate homeostasis [20]. A study found that calcitriol has a beneficial effect on the progression of cancers, autoimmune diseases, and other inflammatory diseases [21,22]. Several epidemiological and preclinical studies found an association between vitamin D status and progression of breast cancer [23], colorectal cancer [24], bladder cancer [25], thyroid cancer [26], and melanoma [27]. Slominski et al also reported that low serum 25 (OH) D3 is associated with reduced survival [28]. Additionally, preclinical studies have found an association between calcitriol metabolites and tumor growth in mouse models. Although the effects on mortality were not significant, mice treated with D3 metabolites were shown to have a better health condition than those receiving placebo [29]. Genomic studies have also shown that low-to-absent expression of VDR and cytochrome p450 27B1 (CYP27B1) was associated with tumor progression and shorter disease-free survival times in melanoma patients [28]. Consistent with their findings, several recent studies have shown the anti-tumor effects of vitamin D in melanoma. VDR polymorphism and expression levels have been shown multiple times to be significantly correlated with melanoma survival or tumor progression [30–32]. Calcitriol via a genomic pathway regulates multiple pathways in inhibiting cancer pathogenesis such as anti-proliferative effect, induction of apoptosis, stimulation of differentiation, anti-inflammatory effect, and inhibition of angiogenesis [10]. Previous in vitro studies revealed that calcitriol affected apoptosis-related proteins such as caspase-3 and poly adenosine diphosphate-ribose polymerases (PARP) protein expressions [33,34]. In the present study, we investigated the anti-proliferative effect and the apoptosis effect of calcitriol. Therefore, we studied the effects of calcitriol on multiple cancer cell lines, especially melanoma, cervical cancer, and breast cancer cells. We also explored the uptake regulation of calcitriol by cancer cells. In addition, the possible involvement of Beclin, PARP, caspase-3, caspase-8, and caspase-9 as apoptotic regulators were also examined, supporting the possibilities of vitamin D as adjuvant therapy of melanoma. The role of calcitriol is not only limited to the calcemic effect, but also has potential anti-cancer effects, including anti-proliferation and apoptosis induction of cancer cells.
Material and Methods
CALCITRIOL:
Calcitriol (1,25(OH)2D3) was purchased from Sigma (catalog number: 32222-06-3, St. Louis, MO, USA). Calcitriol was diluted in 2% ethanol with a ratio of 5 mg/100 μL.
CELL LINES:
B16–F10 cells (melanoma cell line, CRL-6475™), MCF7 cells (breast cancer cell line, HTB-22™), and HeLa cells (cervical cancer cell line, CCL-2™) were obtained from the American Type Culture Collection (ATCC®, Manassas, VA, USA). Throughout the experiment we only used cells at passages 5 and 6 to maintain the characteristics of the cells. Dulbecco’s modified Eagle’s medium (DMEM high glucose) (Gibco 11965092, Waltham, MA, USA) supplemented with 15% fetal bovine serum (FBS) (Gibco 10270106) and 1% penicillin streptomycin (Penstrep) (Gibco 11548876), maintained at 37°C in a humidified incubator with 5% CO2.
CALCITRIOL TREATMENT AND MTS CELL PROLIFERATION ASSAY:
B16–F10 cells (1.7×102 cells/well) were seeded in a 96-well plate in a volume of 100 μL. After 24 h, cells were treated by various concentrations of calcitriol at 125 (0.325 μM), 62.5 (0.1625 μM), 31.25 (0.08 μM), 15.63 (0.04 μM), and 7.81 ppm (0.02 μM). Additionally, 60 ppm (0.19 μM) cisplatin was used as a positive control, ethanol 2% as negative control, DMEM as media only control, and DMEM with cells without any treatments for 24 h. All samples were incubated in Presto blue reagent (ThermoFisher Scientific A13262, Waltham, MA, USA) for 2 h before absorbance measurement at 570 nm. Cell viability was calculated by dividing the absorbance of the sample and control that was already subtracted by control absorbance and multiplied by 100%. The IC50 was analyzed by using 4 parametric non-linear regressions.
CELL TREATMENT FOR REAL-TIME PCR:
About 106 cells were cultured and proliferated in a 24-well plate until 80% confluence, then treated with various doses of calcitriol. After 24-h treatment, media were aspirated, and the adherent cells were washed using ice-cold phosphate-buffered saline (PBS) (Gibco 18912014). TRIzol reagent (ThermoFisher Scientific 15596026) was added 100 μL per well, and the plates were scraped. The TRIzol-cell lysates were aspirated and deposited into a microtube. Twenty-five μL of chloroform (Merck 102445, Burlington, MA, USA) was added, and the tube was shaken vigorously for 15 s. The samples were centrifuged at 10 000 rpm for 5 min. The clear aqueous phase was removed using a micropipette and was separated into another tube, 550 μL of isopropanol (Merck 109634.2500) was added to the aqueous phase and mixed gently. The solution is left at room temperature for 5 min. The solution was centrifuged at 14 000 rpm for at least 20 min. Samples were then placed on ice. The isopropanol was removed, and 1 mL of 75% ethanol in diethyl pyrocarbonate (DEPC)-treated water was added and mixed gently. The solution is recentrifuged at 9500 rpm for 5 min. The ethanol was removed, and the pellets were allowed to air dry, then 15 μL of DEPC-treated water was added to the RNA pellet. The dissolved RNA was then measured using a multimode reader (TECAN M200Pro, Männedorf, Switzerland). The absorbance ratio at 260/280 nm should be 1.8–2.0.
REAL-TIME PCR:
The RNA/primer mixture used in the reaction were: total RNA 1.5 μL, 10 μM forward primer 0.8 μL, and 10 μM reverse primer 0.8 μL. The master mixture (SensiFAST SYBR No-ROX One-Step Kit BIO720005, Bioline, London, UK) was prepared for each reaction and consisted of 2x buffer 10 μL, reverse transcriptase 0.2 μL, inhibitor 0.4 μL, and DEPC-treated water 6.3 μL. The master mix was then added to the RNA/primer mixture and mixed briefly. The thermal cycler (AriaMx, Santa Clara, CA, USA) was set up with the following specification: 45°C for 10 min, 1 cycle; 95°C for 2 min, 1 cycle; 95°C for 5 s 60°C for 10 s, and 72°C for 5 s, for 40 cycles. Specific primers sequences and its annealing conditions are detailed in Supplementary Table 1. Results of real-time PCR were then analyzed using the 2(−ΔΔCt) method.
PROTEIN EXTRACTION AND WESTERN BLOT:
For western blotting, B16–F10 cells were plated in a 24-well plate (5×104 cells/well). After 24 h, cells were treated by 93 (0.24 μM) and 186 ppm (0.48 μM) of calcitriol, and other groups were treated by 60 ppm (0.19 μM) of cisplatin as positive control and ethanol 2% as a negative control for 22-h incubation. The cells were harvested and processed for protein extraction. We added 120 μL/well of lysis solution to a 24-well plate. The lysis solution consisted of a radioimmunoprecipitation assay buffer (RIPA buffer) (ThermoFisher Scientific 89900) and a sample buffer in a ratio of 1: 1. Protein inhibitor (Sigma-Aldrich MSSAFE), and dithiothreitol (DTT) (Promega P1171, Madison, WI, USA) were added later in the ratio of 1: 100. All samples were heated at 96°C for 5 min and snap frozen on ice for 2–3 min. Ten μL of protein was separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 2.5 h. SDS-PAGE was transferred to a nitrocellulose membrane (GE Healthcare 10-6000-02, Chicago, IL, USA) by electrophoresis for 30 min. A 2% blocking reagent that consisted of 0.25% bovine serum albumin (BSA) (Sigma-Aldrich 1.12018) and phosphate-buffered saline Tween-20 (PBST) was added. Membrane immunoblotting was conducted using primary antibodies (Supplementary Table 2), then incubated at 4°C overnight. The signals were imaged by Li-Cor Odyssey CLx (Lincoln, NE, USA). Protein band thickness was analyzed using LI-COR software. Blots were stripped by stripping buffer from ThermoFisher Scientific 21059 and reprobed using β-actin (R&D MAB8929, Minneapolis, MN, USA) as an internal control for protein levels monitoring.
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY FOR MEASURING VITAMIN D UPTAKES:
The standard solution was created using 10 mg of vitamin D dissolved in 10 mL of distilled water to yield 1000 ppm (2.6 μM). One mL of the previous solution was further dissolved in 10 mL of distilled water to generate the final standard solution with a concentration of 100 ppm (0.26 μM). We serially diluted the standard solution to yield different concentrations using 0.5, 1, 1.5, 2, 2.5, and 3 mL of standard solution mixed with 10 mL of ethanol (Merck 100983). Serial concentration of 5 (0.013 μM), 10 (0.026 μM), 15 (0.039 μM), 20 (0.052 μM), 25 (0.065 μM), and 30 ppm (0.078 μM), respectively, were used in this experiment (Supplementary Figure 1). For the sample measurements, 500 μL of the sample medium was added to HPLC-grade methanol (Merck 106007) and vortexed for 1 min. The sample medium was then filtered using a 0.45-μm Millipore filter. The sediment was also mixed with 500 μL of HPLC-grade methanol and was homogenized using a vortex machine for 2 min. The samples were then filtered using a 0.45-μm Millipore filter. The mobile phase used for HPLC was 80% acetonitrile (Merck 100030) and 20% methanol. Vitamin D absorbance was detected at 264 nm wavelength.
APOPTOSIS STAINING WITH ODYSSEY CLX NEAR-INFRARED IMAGING SYSTEM:
Apoptosis staining was done using the Li-Cor Odyssey CLx system following the manufacturer’s recommended protocol outlined in the PSVue 794 reagent kit documentation.
STATISTICAL ANALYSIS:
Data were analyzed by one-way analysis of variance (ANOVA) using SPSS version 20 software and GraphPad Prism version 7 software for Windows and are presented as means±standard error of the mean (SEM). Statistical significance was regarded as
Results
CALCITRIOL INHIBITED CELL PROLIFERATION IN B16–F10 CELLS:
Calcitriol induced alteration of the morphological appearances of cells after treatment with various concentrations of vitamin D for 24 h compared with controls (Figure 1A). Marked cellular morphology changes were shown and indicated cell death. Near-infrared imaging using PSVue to show apoptosis cells can be seen in Figure 1B. The results show comparable apoptosis of cells between calcitriol and cisplatin, confirming the morphological changes shown in Figure 1A. Cell viability assay was done using the resazurin reduction assay, as shown in Figure 1C. Calcitriol showed dose-dependent growth inhibition in B16–F10 cells with a statistically significant decrease in cell viability at doses 125 (0.325 μM), 62.5 (0.1625 μM), and 31.25 (0.08 μM) (P <0.001). The IC50 dose of calcitriol is 93.88 ppm (0.244 μM), as shown in Figure 1D. Additionally, we also observed calcitriol has a potent effect as an anti-tumor agent to induced cell vulnerability in HeLa cell at dose 62.31 ppm (0.19 μM) (Supplementary Figure 2A) and MCF7 at dose 62.93 ppm (0.17 μM) (Supplementary Figure 2B).
CALCITRIOL UPTAKE BY B16–F10 CELLS:
We used high-performance liquid chromatography (HPLC) to measure the concentration of calcitriol in the cell medium. Standardization for calcitriol detection using HPLC is shown in Supplementary Figure 1. We measured calcitriol levels in the culture medium and inside the cells. Figure 2A shows the time-dependent uptake reflected by the level of calcitriol uptake by the B16–F10 cells. We measured the calcitriol uptake by comparing calcitriol level in the medium and inside the cells. Figure 2B shows representative HPLC results of the culture medium. The highest level of calcitriol uptake was at 60 min, with medium concentration decreasing exponentially and was no longer detected after 120 min. Figure 2C shows that the cellular uptake of calcitriol was highest at 30–60 min and metabolized in the cell to an undetectable level at 2 h.
CALCITRIOL UPREGULATED APOPTOSIS-RELATED GENES:
We observed that calcitriol did not upregulate the expression of B cell lymphoma 2 (Bcl2) and Bax genes, unlike cisplatin (Figure 3A). On the other hand, cisplatin upregulated the expression of VDR, RXR alpha, Bcl2, and Bax. However, similar to cisplatin, calcitriol upregulated the expression levels of caspase-3, caspase-8, and caspase-9, reflecting the differential regulation of the apoptosis pathway of calcitriol compared to cisplatin.
CALCITRIOL UPREGULATED THE APOPTOSIS-RELATED PROTEINS WITH COMPARABLE LEVELS TO CISPLATIN:
Western blot results showed that calcitriol increased the protein levels of cleaved caspase-3, caspase-8, caspase-9, Beclin, and PARP to comparable levels with cisplatin, showing active apoptosis activity and involvement of Beclin and PARP activation. Figure 4 presents representative blots comparing the IC50 and 2× IC50 doses of calcitriol compared to negative and positive controls.
Discussion
Cancer cells can modify and suppress apoptosis to supports their survival [35]. Apoptosis is programmed cell death of a multicellular organism to destroy cells that are not needed or are defective [36]. Morphological features of an apoptotic cell are characterized by loss of adherence, cell shrinkage, condensed chromatin and cytoplasm, and surface blebbing [37,38] At the later stage, the cell fragments to form apoptosis bodies [38]. Our result showed a typical round-shaped plasma membrane that indicated early apoptosis morphology (Figure 1A). Colstron et al were the first to demonstrate the presence of VDR in Hs69T cells, a malignant melanoma cell line. Their study also observed that calcitriol inhibited proliferation in a dose-dependent manner [39]. A recent study observed significant inhibition of murine B16–F10 cells under calcitriol treatment [40]. These previous studies support our finding that calcitriol also decreased cell viability starting at a dose of 31.25 ppm (0.08125 μM) and increasing in a dose-dependent fashion (Figure 1C) with highly significant differences compared to control (
We showed that uptake of calcitriol by melanoma cells was highest at 60 min after treatment using HPLC. The uptake is reflected by the exponential decrease in calcitriol concentration inside the medium. Calcitriol is a fat-soluble compound and thus quickly passes through the lipid bilayer to interact with VDR, where it exerts its biological effects. Previous studies reported that calcitriol could activate the caspase-dependent apoptosis pathway [33,41]. However, the mechanism of calcitriol-induced apoptosis in melanoma was less documented. The binding of calcitriol to VDR-RXR is crucial to activating the genomic pathway [9]. Wasiewicz reported that calcitriol increased the VDR levels in pigmented and non-pigmented B16–F10 cells [40]. However, our results did not show a significant increase in VDR gene levels after the application of calcitriol (Figure 3A) due to time of incubation, doses, and the study approach. Nevertheless, it is possible that dose toxicity of calcitriol defines the role of specific mechanism induced in melanoma cells. Our results showed significant upregulation of apoptosis-associated caspases such as caspase-3, caspase-8, and caspase-9 after treatment with calcitriol with specific dose treatment (Figure 4B).
An in vitro experiment using breast cancer and colon cancer cell lines showed the upregulation of pro-apoptosis protein and caspases and downregulation of anti-apoptosis proteins after vitamin D treatment [42]. In addition, novel secosteroids of 20(OH)D3 and 20,23(OH)2D3 show potent anti-melanoma activity in vitro, being excellent candidates for preclinical testing [43].
In line with those reports, we also observed that calcitriol reduced viability and stimulated other cell lines like HeLa and MCF 7, leading to cell death (Supplementary Figure 1). Apoptosis protein imbalance leads to mitochondrial outer membrane permeabilization (MOMP) and mitochondrial apoptosis molecule release [44]. Cytochrome C binds adenosine triphosphate (ATP) or deoxyadenosine triphosphate (dATP) to apoptosis protease activating factor-1 (APAF-1) to form a wheel-like hexamer called apoptosome [38]. Apoptosome binds the initiator caspase, caspase-9 [38]. The cleavage of caspase-9 triggers activation of other procaspases, promoting the executioner caspases: caspase-3, caspase-6, and caspase-7 [45]. Executioner caspase is cleaved, causing cell apoptosis machinery activation [45]. Bao et al observed the effect of calcitriol treatment on BGC-823 cells, a gastric model cell line, showing the upregulation of caspase-3 [33]. A study conducted in another human melanoma cell line, G-361 cells, showed increased caspase-3 and caspase-8 protein levels after calcitriol treatment [46]. Our results showed that cleaved caspase-3, caspase-8, caspase-9 and cleaved PARP levels were increased (Figure 4B), suggesting that active apoptosis occurred in B16–F10 cells after treatment with calcitriol with comparable levels to cisplatin.
Calcitriol also showed different effects toward cancer cells compared to normal cells. Studies using endothelial cells derived from cancer tissues have shown that calcitriol preferentially induces apoptosis in tumor-derived murine endothelial cells [47]. These effects were not seen in normal endothelial cells. In fact, several studies have shown that calcitriol is generally beneficial or has no effects on normal proliferating cells [48,49]. Therefore, these findings support the potential use of calcitriol as a candidate adjuvant for cancer treatment.
Conclusions
Taken together, our data show that the administration of calcitriol induces apoptosis in B16–F10 cells. These findings of apoptosis were verified using cellular morphology, cell viability assay, near-infrared imaging, and the protein expression levels of apoptosis-related proteins. Therefore, calcitriol appears to induce apoptosis in cancer cells and is a potential treatment. However, further research is needed to explore new strategies for use of calcitriol in metastatic melanoma adjuvant therapy.
Figures
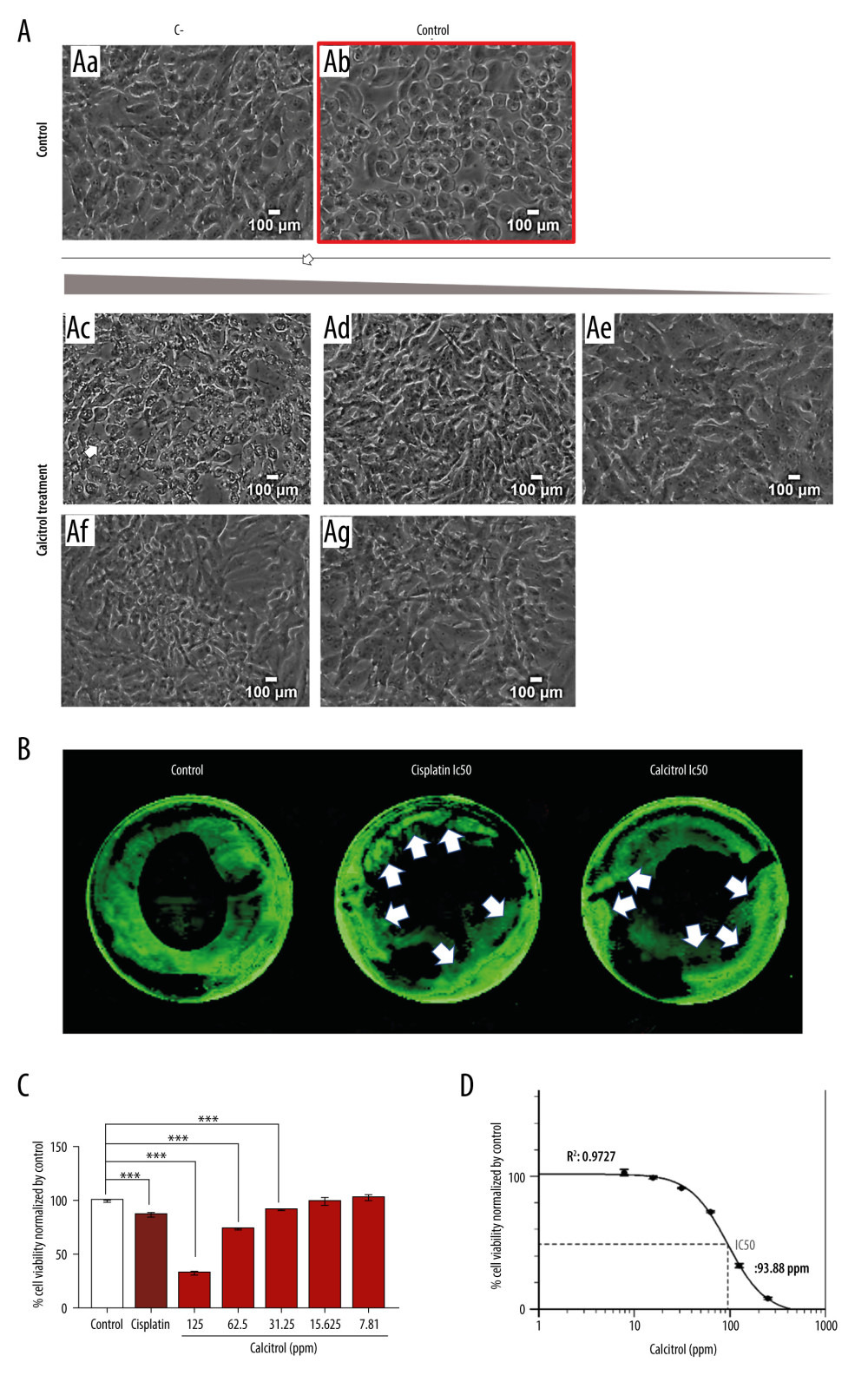 Figure 1. (A) Morphology of B16–F10 cells after 24-h incubation (scale bar=100 μm). Calcitriol is given as follows: ethanol 2% as negative control (Aa), cisplatin as positive control (Ab), calcitriol 125 ppm (0.325 μM) (Ac), 62.5 ppm (0.1625 μM) (Ad), 31.25 ppm (0.08 μM) (Ae), 15.63 (0.04 μM) (Af), 7.81 ppm (Ag). The arrows show the apoptosis cell death morphology. (B) Calcitriol increased the apoptosis process. White arrow points to the green dot accumulation reflecting the apoptosis in the cell. (C) Dose-dependent calcitriol treatment by various ranges of concentrations for 24 h showed inhibition of B16–F10 cells proliferation and decrease in viability at 125 ppm (0.325 μM) and 62.5 ppm (0.1625 μM). (D) IC50 was analyzed by using 4 parametric non-linear regression and plotted in GraphPad Prism. Each data point represents the average of 8 set duplications with SEM. Significance was considered * P<0.05, ** P<0.01, *** P<0.001.
Figure 1. (A) Morphology of B16–F10 cells after 24-h incubation (scale bar=100 μm). Calcitriol is given as follows: ethanol 2% as negative control (Aa), cisplatin as positive control (Ab), calcitriol 125 ppm (0.325 μM) (Ac), 62.5 ppm (0.1625 μM) (Ad), 31.25 ppm (0.08 μM) (Ae), 15.63 (0.04 μM) (Af), 7.81 ppm (Ag). The arrows show the apoptosis cell death morphology. (B) Calcitriol increased the apoptosis process. White arrow points to the green dot accumulation reflecting the apoptosis in the cell. (C) Dose-dependent calcitriol treatment by various ranges of concentrations for 24 h showed inhibition of B16–F10 cells proliferation and decrease in viability at 125 ppm (0.325 μM) and 62.5 ppm (0.1625 μM). (D) IC50 was analyzed by using 4 parametric non-linear regression and plotted in GraphPad Prism. Each data point represents the average of 8 set duplications with SEM. Significance was considered * P<0.05, ** P<0.01, *** P<0.001. 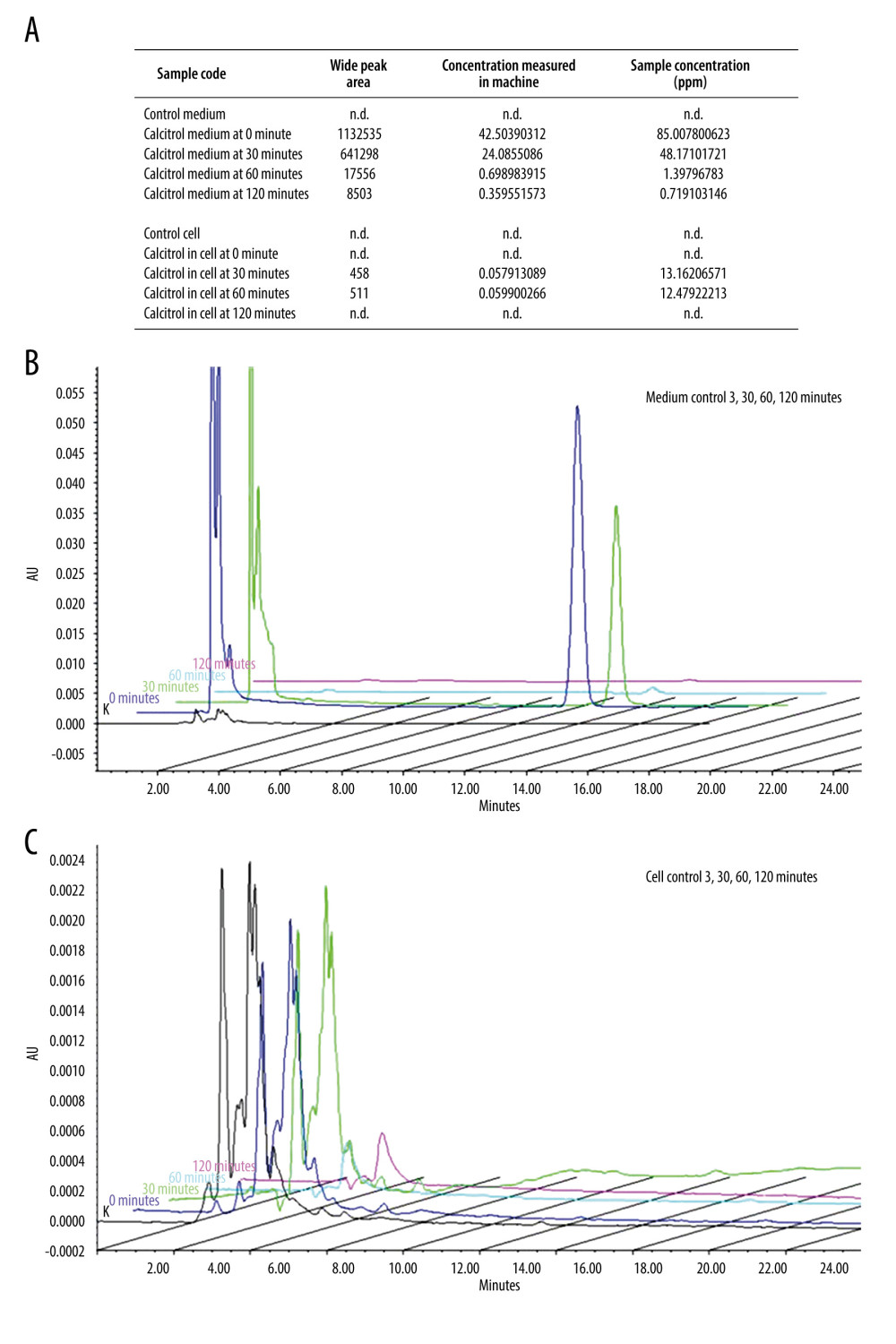 Figure 2. Calcitriol uptake study by B16–F10 cells by HPLC. (A) Detection of calcitriol content after time-dependent treatment in B16–F10 cells and medium. In the medium, calcitriol content was detected at 0 and 30 min. Although low, calcitriol was still detected at 60 and 120 min, while in the cell, the calcitriol content was detected at 30 and 60 min. (B) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium. (C) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium.
Figure 2. Calcitriol uptake study by B16–F10 cells by HPLC. (A) Detection of calcitriol content after time-dependent treatment in B16–F10 cells and medium. In the medium, calcitriol content was detected at 0 and 30 min. Although low, calcitriol was still detected at 60 and 120 min, while in the cell, the calcitriol content was detected at 30 and 60 min. (B) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium. (C) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium. 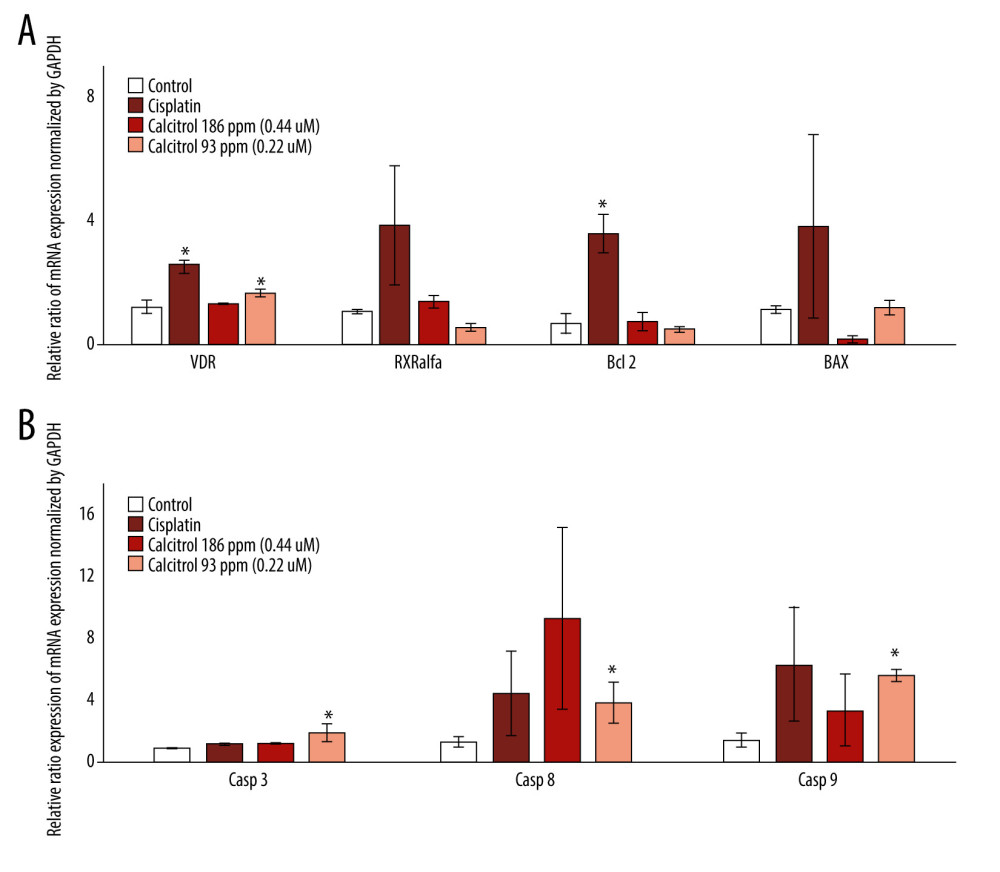 Figure 3. The effect of calcitriol on the gene expression of apoptosis-related proteins using real-time PCR in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative expression was normalized by GAPDH. Caspase-3, caspase-9, and caspase-8 gene expressions were stimulated, but Bax, Beclin, and RXR alpha did not show significant alteration. Data are presented as average mean and SEM. Significance was considered * P<0.05.
Figure 3. The effect of calcitriol on the gene expression of apoptosis-related proteins using real-time PCR in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative expression was normalized by GAPDH. Caspase-3, caspase-9, and caspase-8 gene expressions were stimulated, but Bax, Beclin, and RXR alpha did not show significant alteration. Data are presented as average mean and SEM. Significance was considered * P<0.05. 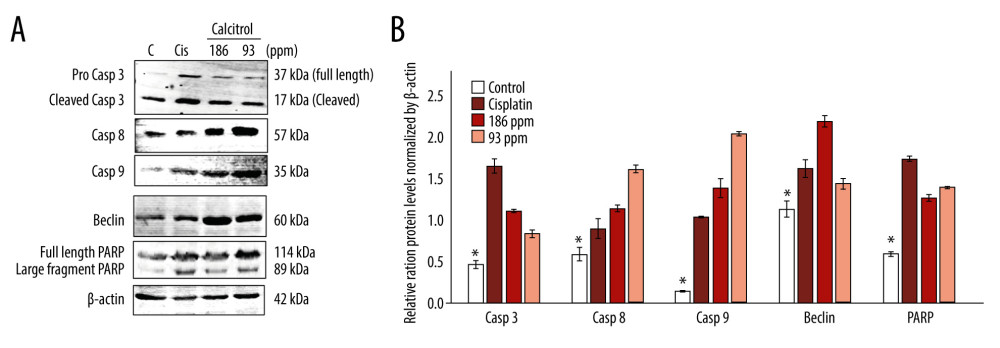 Figure 4. (A, B) The effect of calcitriol on the protein expression of apoptosis-related proteins in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative protein expression was normalized by β-actin. Caspase-3, caspase-9, caspase-8, Beclin, and poly adenosine diphosphate-ribose polymerases (PARP) protein levels were stimulated. Data are presented as average mean and SEM. Significance was considered * P<0.05.
Figure 4. (A, B) The effect of calcitriol on the protein expression of apoptosis-related proteins in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative protein expression was normalized by β-actin. Caspase-3, caspase-9, caspase-8, Beclin, and poly adenosine diphosphate-ribose polymerases (PARP) protein levels were stimulated. Data are presented as average mean and SEM. Significance was considered * P<0.05. References
1. Kanavy HE, Gerstenblith MR, Ultraviolet radiation and melanoma: Semin Cutan Med Surg, 2011; 30; 222-28
2. Andreassi L, UV exposure as a risk factor for skin cancer: Expert Rev Dermatol, 2011; 6; 445-54
3. Ward WH, Farma JM: Cutaneous melanoma: Etiology and therapy, 2017, Brisbane (AU), Codon Publications
4. Reed KB, Brewer JD, Lohse CM, Increasing incidence of melanoma among young adults: An epidemiological study in Olmsted County, Minnesota: Mayo Clin Proc, 2012; 87; 328-34
5. Ali Z, Yousaf N, Larkin J, Melanoma epidemiology, biology and prognosis: EJC Suppl, 2013; 11; 81-91
6. Giammanco M, Di Majo D, Giammanco M, Vitamin D in cancer chemoprevention Vitamin D in cancer chemoprevention: Pharm Biol, 2015; 53; 1399-434
7. Liu Y, Sheikh MS, Melanoma: Molecular pathogenesis and therapeutic management: Mol Cell Pharmacol, 2014; 6; 228
8. Shtivelman E, Davies MQA, Hwu P, Pathways and therapeutic targets in melanoma: Oncotarget, 2014; 5; 1701-52
9. Trump DL, Deeb KK, Johnson CS, Oncology: Recent advances Vitamin D: Considerations in the continued development as an agent for cancer prevention and therapy: Cancer J, 2010; 16; 1-9
10. Feldman D, Krishnan AV, Swami S, The role of vitamin D in reducing cancer risk and progression: Nat Rev Cancer, 2014; 14; 342-57
11. Piotrowska A, Wierzbicka J, Zmijewski M, Vitamin D in the skin physiology and pathology: Acta Biochim Pol, 2016; 63; 17-29
12. Mostafa WZ, Hegazy RA, Vitamin D and the skin: Focus on a complex relationship: A review: J Adv Res, 2015; 6; 793-804
13. Bikle DD, Vitamin D metabolism, mechanism of action, and clinical applications: Chem Biol, 2014; 21; 319-29
14. Carlberg C, Campbell MJ, Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor: Steroids, 2013; 78; 127-36
15. Khan QJ, Kimler BF, Fabian CJ, The relationship between vitamin D and breast cancer incidence and natural history: Curr Oncol Rep, 2010; 12; 136-42
16. Slominski AT, Kim TK, Shehabi HZ, In vivo evidence for a novel pathway of vitamin D metabolism initiated by P450scc and modified by CYP27B1: FASEB J, 2012; 26; 3901-15
17. Slominski AT, Kim TK, Shehabi HZ, In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland: Mol Cell Endocrinol, 2014; 383; 181-92
18. Slominski AT, Kim TK, Li W, Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland: Sci Rep, 2015; 5; 14875
19. Slominski AT, Chaiprasongsuk A, Janjetovic Z, Photoprotective properties of Vitamin D and Lumisterol Hydroxyderivatives: Cell Biochem Biophys, 2020; 78; 165-80
20. Christakos S, Ajibade DV, Dhawan P, Vitamin D: Metabolism: Endocrinol Metab Clin, 2010; 39; 243-53
21. Vuolo L, di somma C, Faggiano A, Colao A, Vitamin D and cancer: Front Endocrin, 2012; 3; 58
22. Kriegel MA, Manson JE, Costenbader KH, Does vitamin D affect risk of developing autoimmune disease?: A systematic review: Semin Arthritis Rheum, 2011; 40; 512-31
23. Hossain S, Beydoun MA, Beydoun HA, Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies: Clin Nutr ESPEN, 2019; 30; 170-84
24. Zhu K, Knuiman M, Divitini M, Lower serum 25-hydroxyvitamin D is associated with colorectal and breast cancer, but not overall cancer risk: A 20-year cohort study: Nutr Res, 2019; 67; 100-7
25. Saiag P, Aegerter P, Vitoux D, Prognostic value of 25-hydroxyvitamin D3 levels at diagnosis and during follow-up in melanoma patients: J Natl Cancer Inst, 2015; 107; djv264
26. Zhao J, Wang H, Zhang Z, Vitamin D deficiency as a risk factor for thyroid cancer: A meta-analysis of case-control studies: Nutrition, 2019; 57; 5-11
27. Slominski AT, Brożyna AA, Skobowiat C, On the role of classical and novel forms of vitamin D in melanoma progression and management: J Steroid Biochem Mol Biol, 2018; 177; 159-70
28. Slominski AT, Brożyna AA, Zmijewski MA, Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management: Lab Investig, 2017; 97; 706-24
29. Skobowiat C, Oak ASW, Kim T-K: Oncotarget, 2017; 8; 9823-34
30. Vasilovici A, Grigore L, Ungureanu L, Vitamin D receptor polymorphisms and melanoma (review): Oncol Lett, 2019; 17; 4162-69
31. Muralidhar S, Filia A, Nsengimana J, Vitamin D – VDR signaling inhibits Wnt/β-catenin – mediated melanoma progression and promotes antitumor immunity: Cancer Res, 2019; 79; 5986-98
32. Slominski AT, Brożyna AA, Skobowiat C, On the role of classical and novel forms of vitamin D in melanoma progression and management: J Steroid Biochem Mol Biol, 2018; 177; 159-70
33. Bao A, Li Y, Tong Y, 1,25-Dihydroxyvitamin D3 and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells: Int J Mol Med, 2014; 33; 1177-84
34. Chaitanya GV, Alexander JS, Babu PP, PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration: Cell Commun Signal, 2010; 8; 31
35. Ghavami S, Hashemi M, Ande SR, Apoptosis and cancer: Mutations within caspase genes: J Med Genet, 2009; 46; 497-510
36. Sendoel A, Hengartner MO, Apoptotic cell death under hypoxia: Physiology, 2014; 29; 168-76
37. Balvan J, Křížová A, Gumulec J, Multimodal holographic microscopy: Distinction between apoptosis and oncosis: PLoS One, 2015; 10; e0121674
38. Kumar V, Abbas AK, Aster JC, Perkins JA: Robbins and cotran pathologic basis of disease, 2015, Canada (US), Elsevier
39. Colston K, Colston MJ, Feldman D, 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture: Endocrinology, 1981; 108; 1083-86
40. Wasiewicz T, Szyszka P, Cichorek M, Antitumor effects of vitamin D analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation: Int J Mol Sci, 2015; 16; 6645-67
41. Fingas CD, Altinbas A, Schlattjan M, Expression of apoptosis-and vitamin D pathway-related genes in hepatocellular carcinoma: Digestion, 2013; 87; 176-81
42. Cao H, Xu Y, de Necochea-Campion R, Application of vitamin D and vitamin D analogs in acute myelogenous leukemia: Exp Hematol, 2017; 50; 1-12
43. Slominski AT, Janjetovic Z, Kim TK, Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes: Anticancer Res, 2012; 32; 3733-42
44. Pfeffer CM, Singh ATK, Apoptosis: A target for anti-cancer therapy: Int J Mol Sci, 2018; 19; 448
45. Green DR, Llambi F, Cell death signaling: Cold Spring Harb Perspect Biol, 2015; 7; a006080
46. Lee JH, Park S, Cheon S, 1,25-Dihydroxyvitamin D3 enhances NK susceptibility of human melanoma cells via Hsp60-mediated FAS expression: Eur J Immunol, 2011; 41; 2937-46
47. Flynn G, Chung I, Yu W-D, Calcitriol (1,25-Dihydroxycholecalciferol) selectively inhibits proliferation of freshly isolated tumor-derived endothelial cells and induces spoptosis: Oncology, 2006; 70; 447-57
48. Bao B-Y, Ting H-J, Hsu J-W, Lee Y-F: Int J Cancer, 2008; 122; 2699-706
49. Fernández-Barral A, Costales-Carrera A, Buira SP, Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids: FEBS J, 2019; 287; 53-72
Figures
 Figure 1. (A) Morphology of B16–F10 cells after 24-h incubation (scale bar=100 μm). Calcitriol is given as follows: ethanol 2% as negative control (Aa), cisplatin as positive control (Ab), calcitriol 125 ppm (0.325 μM) (Ac), 62.5 ppm (0.1625 μM) (Ad), 31.25 ppm (0.08 μM) (Ae), 15.63 (0.04 μM) (Af), 7.81 ppm (Ag). The arrows show the apoptosis cell death morphology. (B) Calcitriol increased the apoptosis process. White arrow points to the green dot accumulation reflecting the apoptosis in the cell. (C) Dose-dependent calcitriol treatment by various ranges of concentrations for 24 h showed inhibition of B16–F10 cells proliferation and decrease in viability at 125 ppm (0.325 μM) and 62.5 ppm (0.1625 μM). (D) IC50 was analyzed by using 4 parametric non-linear regression and plotted in GraphPad Prism. Each data point represents the average of 8 set duplications with SEM. Significance was considered * P<0.05, ** P<0.01, *** P<0.001.
Figure 1. (A) Morphology of B16–F10 cells after 24-h incubation (scale bar=100 μm). Calcitriol is given as follows: ethanol 2% as negative control (Aa), cisplatin as positive control (Ab), calcitriol 125 ppm (0.325 μM) (Ac), 62.5 ppm (0.1625 μM) (Ad), 31.25 ppm (0.08 μM) (Ae), 15.63 (0.04 μM) (Af), 7.81 ppm (Ag). The arrows show the apoptosis cell death morphology. (B) Calcitriol increased the apoptosis process. White arrow points to the green dot accumulation reflecting the apoptosis in the cell. (C) Dose-dependent calcitriol treatment by various ranges of concentrations for 24 h showed inhibition of B16–F10 cells proliferation and decrease in viability at 125 ppm (0.325 μM) and 62.5 ppm (0.1625 μM). (D) IC50 was analyzed by using 4 parametric non-linear regression and plotted in GraphPad Prism. Each data point represents the average of 8 set duplications with SEM. Significance was considered * P<0.05, ** P<0.01, *** P<0.001. Figure 2. Calcitriol uptake study by B16–F10 cells by HPLC. (A) Detection of calcitriol content after time-dependent treatment in B16–F10 cells and medium. In the medium, calcitriol content was detected at 0 and 30 min. Although low, calcitriol was still detected at 60 and 120 min, while in the cell, the calcitriol content was detected at 30 and 60 min. (B) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium. (C) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium.
Figure 2. Calcitriol uptake study by B16–F10 cells by HPLC. (A) Detection of calcitriol content after time-dependent treatment in B16–F10 cells and medium. In the medium, calcitriol content was detected at 0 and 30 min. Although low, calcitriol was still detected at 60 and 120 min, while in the cell, the calcitriol content was detected at 30 and 60 min. (B) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium. (C) Compilation of representative peak curve HPLC of control baseline at 0 min, 30 min, 60 min, and 120 min showed calcitriol content detection inside the medium. Figure 3. The effect of calcitriol on the gene expression of apoptosis-related proteins using real-time PCR in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative expression was normalized by GAPDH. Caspase-3, caspase-9, and caspase-8 gene expressions were stimulated, but Bax, Beclin, and RXR alpha did not show significant alteration. Data are presented as average mean and SEM. Significance was considered * P<0.05.
Figure 3. The effect of calcitriol on the gene expression of apoptosis-related proteins using real-time PCR in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative expression was normalized by GAPDH. Caspase-3, caspase-9, and caspase-8 gene expressions were stimulated, but Bax, Beclin, and RXR alpha did not show significant alteration. Data are presented as average mean and SEM. Significance was considered * P<0.05. Figure 4. (A, B) The effect of calcitriol on the protein expression of apoptosis-related proteins in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative protein expression was normalized by β-actin. Caspase-3, caspase-9, caspase-8, Beclin, and poly adenosine diphosphate-ribose polymerases (PARP) protein levels were stimulated. Data are presented as average mean and SEM. Significance was considered * P<0.05.
Figure 4. (A, B) The effect of calcitriol on the protein expression of apoptosis-related proteins in B16–F10 cells after 24-h treatment. Cells were treated by calcitriol at 93 (0.24 μM) and 186 ppm (0.48 μM), 60 ppm (0.19 mM) of cisplatin as a positive control and negative control. Relative protein expression was normalized by β-actin. Caspase-3, caspase-9, caspase-8, Beclin, and poly adenosine diphosphate-ribose polymerases (PARP) protein levels were stimulated. Data are presented as average mean and SEM. Significance was considered * P<0.05. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176