06 November 2023: Human Study
Urinary Klotho Excretion: A Key Regulator of Sodium Homeostasis in Chronic Kidney Disease Stage 2–4
Po-Jui Chi12ABCDEF, Chung-Jen Lee3BCD, Shih-Yuan HungDOI: 10.12659/MSMBR.942097
Med Sci Monit Basic Res 2023; 29:e942097
Abstract
BACKGROUND: BACKGROUND Soluble α-klotho (klotho) is considered an important regulator of mineral homeostasis in patients with chronic kidney disease (CKD). Since the mineral transport proteins are located on the apical membrane of renal tubular cells, we hypothesized that urine klotho may also be involved in their homeostasis. We aimed to investigate the associations between serum and urine klotho and their impacts on mineral homeostasis in patients with stage 2 to 4 CKD. MATERIAL AND METHODS Serum, spot urine, and 24-h urine of klotho were measured by using enzyme-linked immunosorbent assay. Fractional excretion of sodium, potassium, calcium, phosphate, magnesium, and klotho were calculated. RESULTS A total of 53 patients with CKD stages 2 to 4 were enrolled in this cross-sectional study. The mean age was 71.1±10.5 years, and 68% were men. Linear regression analysis showed that serum log-transformed klotho was negatively associated with log-transformed fractional excretion of klotho (log-FEKlotho) (β=-0.085, P=0.02), showing that urinary klotho excretion could negatively regulate serum klotho levels. Moreover, our multivariate stepwise regression showed log-fractional excretion of sodium was positively associated with log-FEKlotho (β=0.138, P=0.032). This implied urinary klotho excretion positively regulated urinary sodium excretion. CONCLUSIONS Our study showed that urine klotho excretion resulted in decreased serum klotho levels and enhanced urinary sodium excretion in patients with CKD stages 2 to 4. In addition to serum klotho, we found, for the first time, that urine klotho also played a significant role in sodium homeostasis.
MATERIAL AND METHODS:
RESULTS:
CONCLUSIONS:
Keywords: Renal Insufficiency, Chronic, Klotho Protein, Sodium
Background
α-Klotho, an anti-aging protein encoded by the klotho gene, is a 130-kDa transmembrane β-glucuronidase mainly expressed in the renal tubule [1]. Soluble α-klotho (hereafter referred to as klotho), a circulating protein shed from the extracellular domain of membrane form klotho by secretase, is present in the blood, urine, and cerebrospinal fluid [2–4]. Although klotho was found to decrease in patients with early chronic kidney disease (CKD) [5,6], there was no correlation between serum klotho and estimated glomerular filtration rate (eGFR) in patients with stages 2 to 4 CKD [7]. Moreover, Akimoto et al showed that urinary klotho levels, but not serum klotho levels, were linked to functional nephron numbers [3]. Urinary klotho was demonstrated to increase in the first day after cardiac surgery and to be a potential early biomarker for acute kidney injury [8]. Therefore, not only the mechanism for the decline of serum klotho but also the role of urinary klotho in CKD patients remain controversial and in need of investigation.
Mounting evidence shows that klotho can regulate electrolyte homeostasis by modifying the ion transport process. Klotho can prevent endocytosis of transient receptor potential cation channel, subfamily V, member 5 [9,10] and renal outer medullary potassium channel 1 [11] to increase renal calcium reabsorption and potassium excretion in the distal renal tubule. Cooperating with fibroblast growth factor receptor (FGF23), klotho can also promote endocytosis of sodium-dependent phosphate cotransporter type IIa to increase phosphate excretion in the proximal renal tubule [12]. Moreover, klotho deficiency was demonstrated to develop salt-sensitive hypertension in klotho haplo-deficiency mice. An upregulation of thiazide-sensitive sodium chloride cotransporter (NCC) and aldosterone synthesis was also observed in klotho-deficient mice [13,14]. However, all these findings were demonstrated either in vitro or in vivo.
Since all of these ion transport proteins are located on the apical membrane (lumen side) of the renal tubular cells, we hypothesized that urine klotho, instead of serum klotho, impacts renal electrolyte handling. In this study, we aimed to investigate the relationships of either serum or urine klotho on renal electrolyte handling in patients with stages 2 to 4 CKD.
Material and Methods
PATIENTS:
This cross-sectional study was approved by the Institutional Review Board for the Protection of Human Subjects at Dalin Tzu Chi General Hospital in compliance with the Helsinki Declaration (IRB: B10501013). The estimated glomerular filtration rate (eGFR) was calculated by using the Chronic Kidney Disease Epidemiology Collaboration equation [15]. Patients with CKD stages 2 to 4 who visited outpatient clinics of nephrology in Dalin Tzu Chi General Hospital in 2016 were screened, and a total of 53 patients were enrolled. All participants had their blood pressure measured after a 10-min rest by using a standard mercury sphygmomanometer. Patients who experienced episodic acute kidney injury, acute coronary syndrome, aortic dissection, acute cerebrovascular stroke, severe infection, or trauma 3 months before enrollment, or who had a life expectancy of less than 3 months were excluded. Patients who had a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or currently used anti-hypertensive medication(s) were regarded as having hypertension. Patients with a fasting plasma glucose level ≥126 mg/dL or who used glucose-lowering agents were regarding as having diabetes mellitus. The use of angiotensin converting enzyme inhibitor/angiotensin II receptor blockers, calcium channel blockers, and diuretics in patients was recorded.
LABORATORY PARAMETERS:
All the fasting blood, spot urine, and 24-h urine specimens were collected between 7 AM and 10 AM for analysis. Blood samples were immediately centrifuged at 3000×
We calculated the fractional excretion of each electrolyte, such as sodium, potassium, calcium, phosphate, and magnesium, with the following formula: FEX = [(urine X) × (serum creatinine)]/[(serum X) × (urine creatinine)] (where X represents the electrolyte). Fractional excretion of klotho (FEKlotho) was calculated with the following formula: FEKlotho = [(urine klotho) × (serum creatinine)]/[(serum klotho) × (urine creatinine)].
STATISTICAL ANALYSIS:
Continuous data were evaluated with the Kolmogorov-Smirnov test for normal distribution before statistical testing. Categorical data were expressed as numbers and percentages (%). Continuous data normally distributed were expressed as mean±standard deviation, or for others were expressed as median and 25th–75th interquartile range (IQR 25–75). The one-way analysis of variance (ANOVA) test and Kruskal-Wallis H test were used for differences across CKD stages in parametric and nonparametric continuous variables, respectively. The Pearson chi-square test was used for categorical variables. Pearson correlation was used for parametric continuous variables and the Spearman correlation was used for non-parametric or categorical variables. Because klotho, FEKlotho, urine protein-to-creatinine ratio (UPCR), fractional excretion of sodium (FENa), 24-h urine protein, uric acid, fractional excretion of calcium (FECa), C-terminal and intact FGF23, and fractional excretion of potassium (FEK) were skewed, natural logarithmic(ln) transformations were performed to achieve normality. If correlation analyses were statistically significant for klotho, FEKlotho, and FENa, further univariate linear regression analyses were performed. Variables significantly correlated in univariate regression were selected for multivariate stepwise analyses. A
Results
CHARACTERISTICS OF PATIENTS WITH CKD:
Demographic data of these 53 patients, stratified by CKD stages, are shown in Table 1. Among them, 68% were men and the mean age was 71.1±10.5 years. Mean eGFR was 45.5±15.2 ml/min/1.73 m2 and mean 24-h urine protein was 226.8 mg (25th–75th percentage 114.4–838.6 mg). FENa, FEK, fractional excretion of phosphate (FEPi), fractional excretion of magnesium (FEMg), serum intact parathyroid hormone, and cFGF23 were increased, while Hb and 25 hydroxyvitamin D were decreased in patients with advanced CKD. FECa revealed a trend to decline in advanced CKD, but this trend was insignificant. Serum klotho, intact FGF23, and 24-h urine klotho excretion show no differences.
CORRELATIONS BETWEEN SERUM KLOTHO AND OTHER PARAMETERS IN CKD:
When we examined the correlations between serum klotho and other parameters in these patients, our data showed that serum log-klotho was positively correlated with Hb (β=0.271, P=0.049) and negatively correlated with log-uric acid (β=−0.311, P=0.024) and log-FEKlotho (β=−0.352, P=0.01). We performed univariate linear regression analyses by adopting serum log-klotho as a dependent variable and found log-FEKlotho remained the only predictor of serum log-klotho (β=−0.085, P=0.02; Table 2). Our findings suggested that serum klotho level was negatively regulated by FEKlotho in CKD patients, indicating that an increase in urinary klotho excretion resulted in a decline of serum klotho levels.
CORRELATIONS BETWEEN FENA AND CLINICAL PARAMETERS:
Furthermore, we also conducted correlation analyses between FENa and other parameters. Correlation analysis shows FENa was positively correlated with FEK (β=0.66, P<0.0001), FECa (β=0.344, P=0.012), FEPi (β=0.692, P<0.0001), FEMg (β=0.766, P<0.0001), cFGF23 (β=0.377, P=0.005), FEKlotho (β=0.48, P<0.0001), and UPCR (β=0.318, P=0.02), while negatively correlated with eGFR (β=−0.528, P<0.0001), Hb (β=−0.464, P=0.00047) (data are not shown). The results of univariate and multivariate analyses with log-FENa as a dependent variable are shown in Table 3. Multivariate stepwise regression after adjustment of eGFR, Hb, log-FEK, log-FECa, FEPi, FEMg, log-UPCR, log-cFGF23, and log-FEKlotho showed log-FENa was positively correlated with log-FEKlotho (β=0.138, P=0.032), FEPi (β=0.027, P=0.002), and FEMg (β=0.133, P<0.0001). FENa was positively regulated by FEKlotho, suggesting that an increase in urinary klotho excretion can enhance urinary sodium wasting.
Discussion
The findings from our cross-sectional study in patients with CKD stages 2 to 4 are summarized as followed. First, serum klotho is inversely correlated with FEKlotho, indicating the increase in urinary klotho excretion can result in a decline of serum klotho levels. Second, FENa is positively correlated with FEKlotho, implying that an increase in urinary klotho excretion can enhance urinary sodium wasting.
In agreement with our findings in CKD patients, Hu et al found a negative correlation between FEKlotho and serum klotho levels in their animal studies. They demonstrated that the kidney regulates serum klotho levels either by shedding klotho from the renal tubule into the blood or by transcytosis from the basolateral to the apical side of the renal proximal tubule [2], indicating that the kidney is responsible not only for klotho production but also for klotho clearance from circulation.
Many studies have shown that circulating klotho decreased in advanced CKD [5,6,16–18]. However, in contrast, there was no decrease in serum klotho in our CKD patients. In addition, Seiler et al [7] also failed to find this association in advanced CKD. Accordingly, we hypothesized that there are many other factors beyond eGFR, such as renal tubule dysfunction, daily protein excretion, and electrolyte imbalance, that can also influence serum klotho level.
The regulation of sodium excretion was achieved by controlling transporters in renal tubules. NCC is a major sodium transporter channel located in the apical membrane of the distal renal tubule [19]. The abundance and expression of NCC in the apical membrane results in sodium reabsorption. Zhou et al showed that the expression of NCC was upregulated in klotho mutant heterozygous mice rather than in the wild-type mice. Although the functional changes of NCC were not assessed in their study, their finding still implied that klotho repressed the expression of NCC and therefore led to urine sodium wasting [13]. Our study, for the first time, demonstrated the relationship between urine klotho and urinary sodium excretion. However, the mechanism of how klotho impacts NCC still needs to be elucidated. Another study by Zhou et al showed klotho gene deficiency can also upregulate CYP11B2 expression in the adrenal cortex, thereby increasing aldosterone synthesis. Aldosterone can further enhance sodium reabsorption via the decreased abundance of NCC [14].
To the best of our knowledge, the present study is the first study to show that FEKlotho, as a surrogate of urine klotho elimination, is associated with urine sodium excretion in CKD patients. Still, this study had several limitations. First, to investigate the impact of eGFR on serum klotho level, we failed to include patients with all stages of CKD and healthy individuals as control. Second, the single-center study design and a limited number of patients create selection bias in our study. Third, we did not evaluate the dietary phosphate, calcium, and sodium content in our patients.
Conclusions
In conclusion, our study indicated that FEKlotho is negatively correlated with serum klotho levels and positively correlated with FENa, indicating an increase in urinary klotho excretion can result in a decline of serum klotho levels and an increase in sodium wasting. These findings imply that urine excretion of klotho determines both klotho and sodium homeostasis in patients with stages 2 to 4 CKD.
Tables
Table 1. Clinical and laboratory characteristics of patients with chronic kidney disease stratified by disease stage.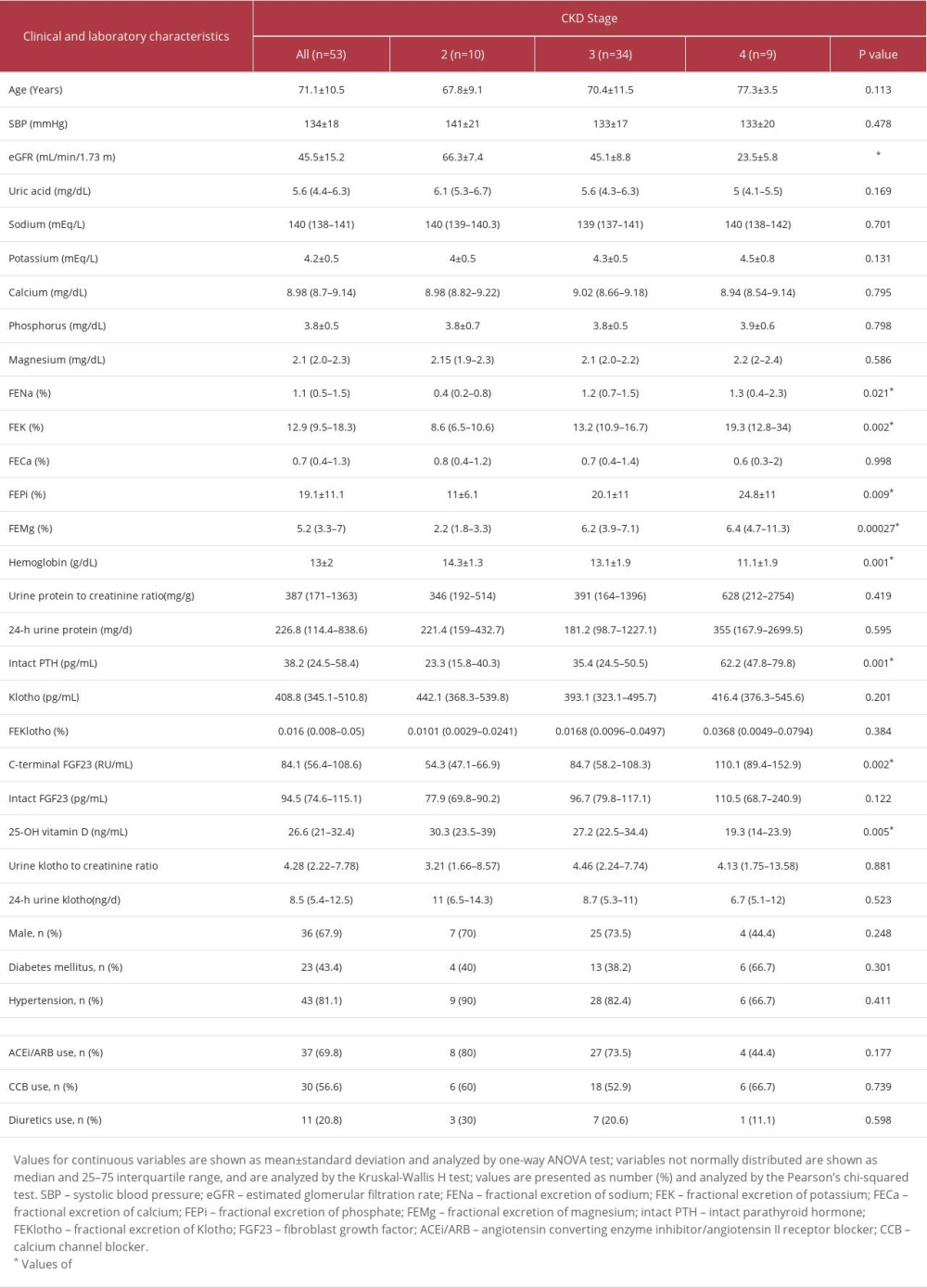 Table 2. Linear regression of log-klotho in patients with chronic kidney disease.
Table 2. Linear regression of log-klotho in patients with chronic kidney disease.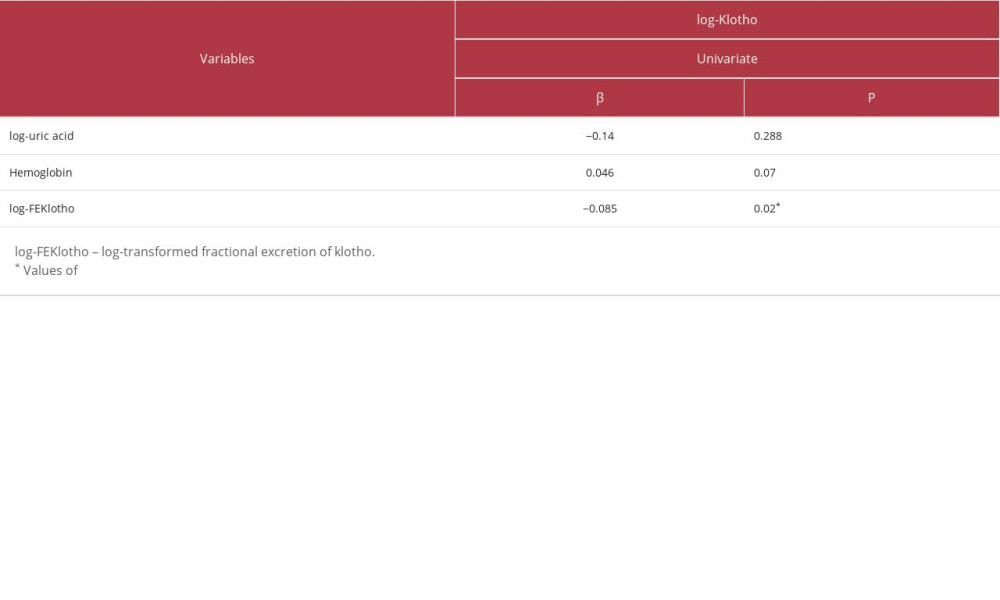 Table 3. Univariate and multivariate stepwise linear regression of log-FENa in patients with chronic kidney disease.
Table 3. Univariate and multivariate stepwise linear regression of log-FENa in patients with chronic kidney disease.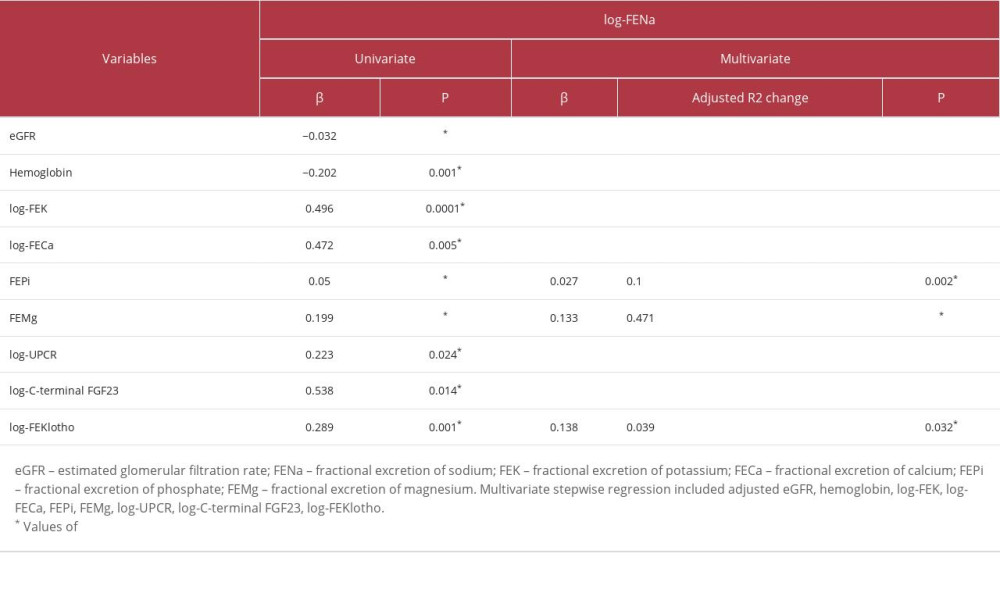
References
1. Kuro-o M, Matsumura Y, Aizawa H, Mutation of the mouse klotho gene leads to a syndrome resembling ageing: Nature, 1997; 390(6655); 45-51
2. Hu MC, Shi M, Zhang J, Renal production, uptake, and handling of circulating αKlotho: J Am Soc Nephrol, 2016; 27(1); 79-90
3. Akimoto T, Yoshizawa H, Watanabe Y, Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease: BMC Nephrol, 2012; 13; 155
4. Semba RD, Moghekar AR, Hu J, Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease: Neurosci Lett, 2014; 558; 37-40
5. Pavik I, Jaeger P, Ebner L, Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study: Nephrol Dial Transplant, 2013; 28(2); 352-59
6. Shimamura Y, Hamada K, Inoue K, Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis: Clin Exp Nephrol, 2012; 16(5); 722-29
7. Seiler S, Wen M, Roth HJ, Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease: Kidney Int, 2013; 83(1); 121-28
8. Qian Y, Che L, Yan Y, Urine klotho is a potential early biomarker for acute kidney injury and associated with poor renal outcome after cardiac surgery: BMC Nephrol, 2019; 20(1); 268
9. Cha SK, Ortega B, Kurosu H, Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1: Proc Natl Acad Sci USA, 2008; 105(28); 9805-10
10. Chang Q, Hoefs S, van der Kemp AW, Topala CN, The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel: Science, 2005; 310(5747); 490-93
11. Cha SK, Hu MC, Kurosu H, Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho: Mol Pharmacol, 2009; 76(1); 38-46
12. Hu MC, Shi M, Zhang J, Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule: FASEB J, 2010; 24(9); 3438-50
13. Zhou X, Chen K, Lei H, Sun Z, Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation: J Am Soc Nephrol, 2015; 26(1); 121-32
14. Zhou X, Chen K, Wang Y, Antiaging gene klotho regulates adrenal CYP11B2 expression and aldosterone synthesis: J Am Soc Nephrol, 2016; 27(6); 1765-76
15. Levey AS, Stevens LA, Schmid CHCKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), A new equation to estimate glomerular filtration rate: Ann Intern Med, 2009; 150(9); 604-12 [Erratum in: Ann Intern Med. 2011;155(6):408]
16. Kim HR, Nam BY, Kim DW, Circulating α-klotho levels in CKD and relationship to progression: Am J Kidney Dis, 2013; 61(6); 899-909
17. Rotondi S, Pasquali M, Tartaglione L, Soluble alpha-Klotho serum levels in chronic kidney disease: Int J Endocrinol, 2015; 2015; 872193
18. Kim SS, Song SH, Kim IJ, Decreased plasma α-Klotho predict progression of nephropathy with type 2 diabetic patients: J Diabetes Complications, 2016; 30(5); 887-92
19. Garg LC, Knepper MA, Burg MB, Mineralocorticoid effects on Na-K-ATPase in individual nephron segments: Am J Physiol, 1981; 240(6); F53-44
Tables
 Table 1. Clinical and laboratory characteristics of patients with chronic kidney disease stratified by disease stage.
Table 1. Clinical and laboratory characteristics of patients with chronic kidney disease stratified by disease stage. Table 2. Linear regression of log-klotho in patients with chronic kidney disease.
Table 2. Linear regression of log-klotho in patients with chronic kidney disease. Table 3. Univariate and multivariate stepwise linear regression of log-FENa in patients with chronic kidney disease.
Table 3. Univariate and multivariate stepwise linear regression of log-FENa in patients with chronic kidney disease. Most Viewed Current Articles
15 Jun 2022 : Clinical Research
Evaluation of Apical Leakage After Root Canal Obturation with Glass Ionomer, Resin, and Zinc Oxide Eugenol ...DOI :10.12659/MSMBR.936675
Med Sci Monit Basic Res 2022; 28:e936675
07 Jul 2022 : Laboratory Research
Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis S...DOI :10.12659/MSMBR.936683
Med Sci Monit Basic Res 2022; 28:e936683
01 Jun 2022 : Laboratory Research
Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Biocerami...DOI :10.12659/MSMBR.936319
Med Sci Monit Basic Res 2022; 28:e936319
08 Dec 2022 : Original article
Use of Estimated Glomerular Filtration Rate and Urine Albumin-to-Creatinine Ratio Based on KDIGO 2012 Guide...DOI :10.12659/MSMBR.938176
Med Sci Monit Basic Res 2022; 28:e938176








